Overview
Annually, approximately 50,000 individuals are diagnosed with head and neck cancer in the United States, and over 11,000 die of the disease. Most patients with head and neck cancer have metastatic disease at the time of diagnosis (regional nodal involvement in 43% and distant metastasis in 10%).
Head and neck cancers encompass a diverse group of uncommon tumors that frequently are aggressive in their biologic behavior. Moreover, patients with a head and neck cancer often develop a second primary tumor, particularly those with a history of smoking.
These tumors occur at an annual rate of 3% to 7%, and 50% to 75% of such new cancers occur in the upper aerodigestive tract or lungs. The incidence of tobacco-related head and neck cancer is decreasing. However, the incidence of cancer due to the human papillomavirus (HPV) continues to increase at a rate of 2% to 4% per year.
The anatomy of the head and neck is complex and is divided into sites and subsites (Figure 1). Tumors of each site have a unique epidemiology, anatomy, natural history, and therapeutic approach. This chapter will review these lesions as a group and then individually by anatomic site.
Epidemiology
Gender
Head and neck cancer is more common in men, with 66% to 95% of cases by site occurring in men. The incidence by gender varies with anatomic location and has been changing as the number of female smokers has increased. The male-female ratio is currently 3:1 for oral cavity and pharyngeal cancers. In patients with Plummer-Vinson syndrome, the ratio is reversed, with 80% of head and neck cancers occurring in women.
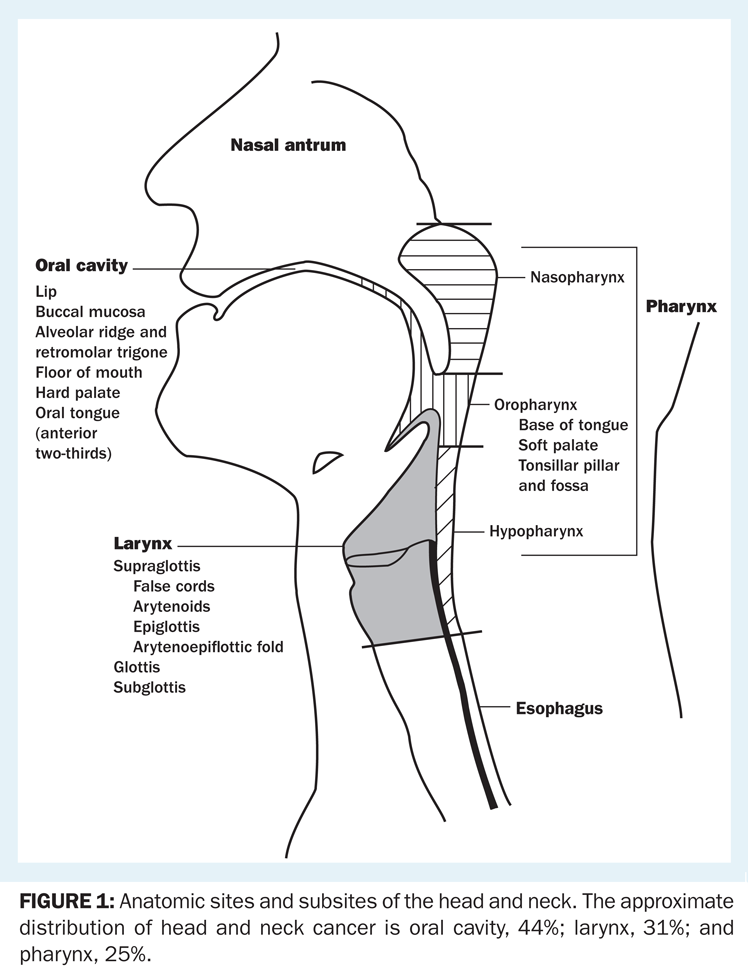
Anatomic sites and subsites of the head and neck. The approximate distribution of head and neck cancer is oral cavity, 44%; larynx, 31%; and pharynx, 25%.
Age
The incidence of head and neck cancer increases with age, especially after 50 years of age. Although most patients are between 50 and 70 years old, younger patients can develop head and neck cancer. There are more women and fewer smokers in the younger patient group. Patients with HPV-associated head and neck cancer tend to be younger, and may be in their 30s or 40s when diagnosed with this disease. It is controversial whether head and neck cancer is more aggressive in younger patients or in older individuals. This “aggressiveness” probably reflects the common delay in diagnosis in the younger population, since, in most studies, younger patients do not have a worse prognosis than their comparable older counterparts.
Race
The incidence of laryngeal cancer is higher in African Americans relative to the white, Asian, and Hispanic populations.
In addition, in African Americans, head and neck cancer is associated with lower survival for similar tumor stages. The overall 5-year survival rate is 56% in whites and 34% in African Americans.
Geography
There are wide variations in the incidence of head and neck cancer among different geographic regions. The risk of laryngeal cancer, for example, is two to six times higher in Bombay, India, than in Scandinavia. The higher incidence of the disease in Asia is thought to reflect the prevalence of risk factors, such as betel nut chewing and use of smokeless tobacco (snuff). In the United States, the high incidence among urban males is thought to reflect exposure to tobacco and alcohol. Among rural women, there is an increased risk of oral cancer related to the use of smokeless tobacco.
Nasopharyngeal carcinoma is another head and neck tumor with a distinct ethnic predilection. Endemic areas include southern China, northern Africa, and regions of the far Northern Hemisphere—areas in which the diet of inhabitants includes large quantities of salted meat and fish. When people from these regions migrate to areas with a lower disease incidence, their risk falls but remains elevated. Cancer of the nasopharynx in these geographic areas also has been associated with Epstein-Barr virus (EBV) infection (see section on “Etiology and risk factors”).
Etiology and Risk Factors
Risk factors for head and neck cancer include tobacco and alcohol use, ultraviolet (UV) light exposure, viral infection, and environmental exposures.
Tobacco
The incidence of head and neck tumors correlates most closely with the use of tobacco. Smoking increases the risk of death by 1% per pack-year. Smoking during treatment with radiation increases the risk of death two-fold.
Cigarettes
Head and neck tumors occur six times more often among cigarette smokers than nonsmokers. The age-standardized risk of mortality from laryngeal cancer appears to rise linearly with increasing cigarette smoking. For the heaviest smokers, death from laryngeal cancer is 20 times more likely than for nonsmokers. Furthermore, active smoking by head and neck cancer patients is associated with significant increases in the annual rate of second primary tumor development (compared with former smokers or those who have never smoked). Use of unfiltered cigarettes or dark, air-cured tobacco is associated with further increases in risk.
Cigars
Often misperceived as posing a lower health risk than cigarette smoking, cigar smoking results in a change in the site distribution for aerodigestive tract cancer, according to epidemiologic data. Although the incidence of cancer at some sites traditionally associated with cigarette smoking (eg, larynx, lungs) is decreased in cigar smokers, the incidence of cancer is actually higher at other sites where pooling of saliva and associated carcinogens tends to occur (oropharynx, esophagus).
Smokeless tobacco
Use of smokeless tobacco also is associated with an increased incidence of head and neck cancer, especially in the oral cavity. Smokeless tobacco users frequently develop premalignant lesions, such as oral leukoplakia, at the site where the tobacco quid rests against the mucosa. Over time, these lesions may progress to invasive carcinomas. The use of snuff has been associated with an increase in cancers of the gum and oral mucosa.
Alcohol
Alcohol consumption, by itself, is a risk factor for the development of pharyngeal and laryngeal tumors, although it is a less potent carcinogen than tobacco. For individuals who use both tobacco and alcohol, these risk factors appear to be synergistic and result in a multiplicative increase in risk.
UV Light Exposure
Exposure to UV light is a risk factor for development of cancer of the lips. At least 33% of patients with lip cancer have outdoor occupations.
Occupational Exposures
A small group of head and neck cancers may be attributable to occupational exposures. Nasal cancer has been associated with wood dust exposure, and squamous cell cancer of the maxillary sinus has been linked to nickel exposure. Petroleum exposure may be associated with pharyngeal cancer, but the relationship has not been proven.
Radiation Exposure
Exposure to radiation is clearly an important risk factor for thyroid cancer and has been associated with cancer of the salivary glands.
Viruses
There is a strong link between EBV exposure and the development of nasopharyngeal cancer. The etiologic role of HPV in oropharyngeal cancer is supported by a growing body of evidence. A case-control study of 100 patients with squamous cancer of the oropharynx documented that HPV DNA type 16 was found in 72% of tumor specimens. Further, 64% of the patients had antibodies to HPV-16 oncoproteins. Oral infection with HPV increased the risk of oropharyngeal cancer with an odds ratio of 14.6 (95% confidence interval [CI], 6.3 to 36.6). This risk was independent of tobacco and alcohol use. These patients appear to have a better outcome following surgery, radiotherapy, and chemotherapy. In addition, these patients have a lower incidence of second cancers and a lower risk of death from other tobacco-related illnesses (eg, heart and lung disease). The development of oropharyngeal cancer was associated with high-risk sexual behaviors, including a higher lifetime number of sexual partners, engagement in oral sex, casual sexual relations, young age at first intercourse, and infrequent condom use. It is hoped than an HPV vaccine will reduce the incidence of oropharyngeal cancer.
Diet
Epidemiologic studies suggest that dietary intake of vitamin A, β-carotene, and α-tocopherol may reduce the risk of developing head and neck cancer.
Marijuana
Smoking marijuana has been associated with development of head and neck cancer, but the degree of risk is unknown.
Anatomy
The anatomy of the head and neck region is complex. The anatomic sites are illustrated in Figure 1. More detailed descriptions are included below in the discussions of specific sites and subsites.
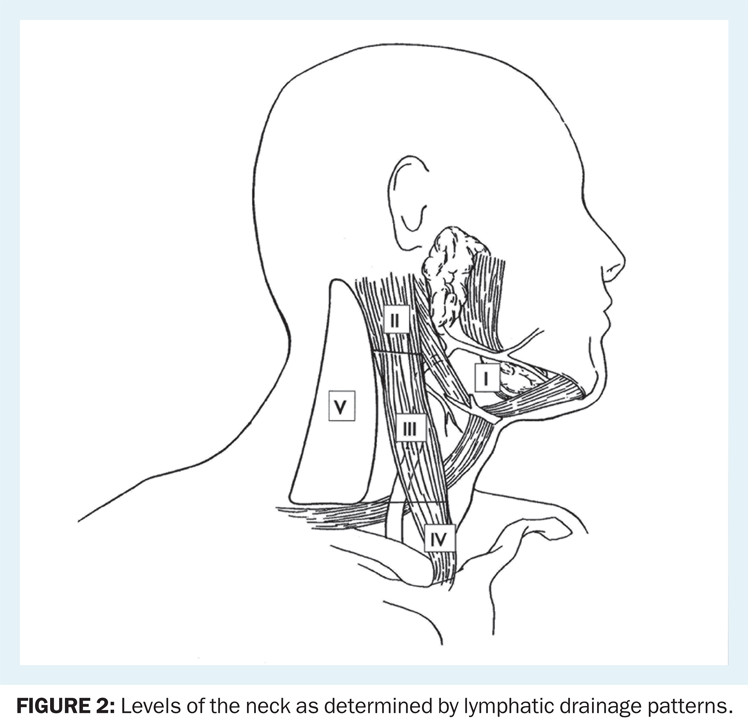
Levels of the Neck
The anatomy of the neck is relevant to the treatment of all head and neck cancers. The neck may be divided into levels (Figure 2). The lymphatic drainage of the unmanipulated neck is systematic and predictable; knowledge of these drainage patterns assists the clinician in locating the primary tumor that has given rise to a neck metastasis (Table 1).
Signs and Symptoms
Head and neck cancer typically produces symptoms referable to the upper aerodigestive tract, including alterations in deglutition, phonation, hearing, and respiration. In particular, patients should be questioned about dysphagia, odynophagia, globus sensation, hoarseness, a change in the ability to form words, epistaxis, epiphora, otalgia, hemoptysis, stuffiness of the ears, and trismus. (Signs and symptoms of cancer at specific anatomic sites and subsites can be found in the respective discussions of these tumors.)
It is important to ascertain the duration and course (progression or improvement) of symptoms. Progression of disease is often noted during the evaluation and worsens the prognosis.
Screening and Diagnosis
Screening
Since many patients with head and neck cancer are unlikely to be engaged in the healthcare system, the means by which patient screening would be achieved remains a fundamental problem.
Diagnosis
The need for expeditious diagnosis of head and neck cancer and referral to skilled head and neck specialists cannot be overemphasized, as early diagnosis can lead to a reduction in mortality. One study suggested that in the 24 months prior to the diagnosis of head and neck cancer, patients had a median of 10.5 healthcare visits. These visits could have provided an opportunity to evaluate patients’ symptoms and underscore the important role of dentists and primary care physicians in the early diagnosis of head and neck cancer.
History
Risk factors as outlined previously, including a history of tobacco and alcohol use and environmental exposures, should be reviewed. Any adult patient with symptoms referable to the upper aerodigestive tract that have lasted longer than 2 weeks or with an asymptomatic neck mass should undergo a thorough examination with a high index of suspicion for carcinoma.
Physical examination
The physical examination is the best means of detecting lesions of the upper aerodigestive tract. Frequently, the initial assessment also will indicate the severity and chronicity of the disease. Due to the frequent occurrence of multiple primary tumors in patients with a head and neck tumor, careful evaluation of the entire upper aerodigestive tract is necessary at the time of diagnosis. The examination should always follow a systematic approach.
Skin/scalp. A search should be made for ulcers, nodules, and pigmented or other suspicious lesions. This part of the evaluation is frequently overlooked.
Cranial nerves. A cranial nerve evaluation is essential for any patient with a head and neck tumor or neck mass (which may be a manifestation of occult cancer). This evaluation should include assessing eye motion (cranial nerves [CN] III, IV, and VI); testing sensation of the face (CN V); examining the muscles of facial expression by having the patient grin, grimace, raise eyebrows, close eyes tightly, show teeth, and puff out the cheeks (CN VII); testing of hearing (CN VIII); assessing gag reflex (CN IX); evaluating vocal cord mobility (CN X); and having the patient fully abduct the shoulder (CN XI) and protrude the tongue (CN XII). Even the slightest abnormality may be helpful in identifying a primary tumor.
Eyes/ears/nose. The eyes, ears, and nose should be evaluated for any sign of mass effect, abnormal drainage/discharge, bleeding, or effusion.
Oral cavity. Halitosis may be the first indication of a lesion in the upper aerodigestive tract. The teeth, gingivae, and entire mucosal surface should be inspected. (Dentures should be removed.) The lymphoid tissue of the tonsillar pillars should be inspected and any asymmetry noted. Tongue mobility also should be evaluated.
The floor of the mouth, tongue, and cheeks should be palpated using a bimanual technique (one gloved finger inside the mouth and the second hand under the mandible). Palpation should be the last step of the examination due to stimulation of the gag reflex. Worrisome lesions should be biopsied.
Neck. A systematic examination of the neck consistently documents the location of any mass. Palpation is the cornerstone of the examination. It is performed by grasping the tissue and feeling the nodes between the thumb and long fingers. The relationship of a mass to major structures, such as the salivary gland, thyroid, and carotid sheath, should be considered.
Important qualities of a mass include location, character, tenderness, size, mobility, and associated thrill or bruit. The thyroid should be palpated.
Laryngoscopy. The nasopharynx, hypopharynx, and larynx should all be examined with care. The vocal cords should be visualized and their mobility evaluated. Mirror examination provides an overall impression of mobility and asymmetry, which may point to a hidden tumor. Nasopharyngoscopes permit a thorough inspection of the upper aerodigestive tract in the office setting. Attention should be focused individually on the piriform sinuses, tongue base, pharyngeal walls, epiglottis, arytenoids, and true and false vocal cords. Also, any pooling of secretions should be noted.
Examination under anesthesia with endoscopy. Approximately 5% of patients with head and neck cancer have a synchronous primary squamous cell cancer of the head and neck, esophagus, or lungs. Examination with the patient under anesthesia with endoscopy (which may include direct laryngoscopy, esophagoscopy, and bronchoscopy) and directed biopsy should be performed in all patients with an occult primary squamous cell cancer and in many patients with a known head and neck primary. Examination with the patient under anesthesia also can provide information regarding the extent of the tumor.
The most common sites of silent primary tumors are the tonsils, base of the tongue, and piriform sinuses. Tumors of the nasopharynx have become easier to identify with the increased use of flexible nasopharyngoscopy. Biopsies should be performed in common areas of silent primaries in addition to the primary anatomic sites associated with lymphatic drainage of any neck mass.
Laboratory evaluation
There are no specific screening laboratory tests other than preoperative studies performed in the diagnostic evaluation of most head and neck carcinomas. EBV, anticapsid antibodies, and serum immunoglobulin G are tumor markers for nasopharyngeal carcinomas.
Diagnostic imaging
Plain x-rays. Posteroanterior and lateral chest x-rays should be obtained in all adult patients to eliminate the possibility of occult lung metastasis or a second primary. A Panorex film may be helpful in delineating bony involvement in some cases of oral cavity lesions.
CT. The CT scan is probably the single most informative test in the assessment of a head and neck tumor. It may delineate the extent of disease and the presence and extent of lymphatic involvement. CT offers high spatial resolution; discriminates among fat, muscle, bone, and other soft tissues; and surpasses MRI in the detection of bony erosion. CT scans of the chest, abdomen, and pelvis sometimes may identify the site of an occult primary tumor presenting with a node low in the neck from lung and intra-abominal primary cancers (for left low neck presentations) if none has been identified. The value of including an abdominal and pelvic CT scan to look for distant metastatic disease in the absence of metastatic lung involvement is minimal.
MRI. An MRI may provide accurate information regarding the size, location, and soft-tissue extent of tumor. It provides limited information regarding bony involvement, unless there is gross involvement of the marrow space. Relatively greater sensitivity of MRI in relation to CT is offset by its decreased specificity. The main disadvantage of MRI is movement artifact, which is a particular problem in the larynx and hypopharynx. Gadolinium-enhanced MRI is probably superior to CT for imaging tumors of the nasopharynx and oropharynx.
PET. PET imaging has been evaluated in both primary and recurrent squamous cell carcinomas of the head and neck. 18F-fluorodeoxyglucose (FDG) is the most commonly used PET radiotracer. It enters the cell and undergoes the first step in glycolysis to produce FDG-6-phosphate, which reflects the metabolic rate of the tissue. The metabolic rate of malignancies is higher than that of most benign tumors or normal tissues. FDG imaging therefore has the potential to distinguish between benign and malignant processes, grade tumors, identify metastases, and diagnose tumor recurrence. In head and neck cancer, FDG imaging is capable of detecting clinically occult recurrences and in determining residual disease in the neck following definitive radiotherapy. It has proved less useful in identifying an occult primary site in the setting of a patient with a squamous cell carcinoma of an unknown primary site as well as evaluating for distant metastatic disease if conventional imaging of the lung does not show distant spread. PET imaging is currently most useful when registered with a CT scan (PET/CT).
In a multicenter, prospective study, published in 2010 by Lonneux et al, involving patients with newly diagnosed and untreated head and neck cancers, PET scanning results were discordant with standard imaging (eg, CT scan, MRI) in 43% of cases, and they altered the therapeutic plan in 14% of patients. Nuanced judgment in adding PET scanning to conventional imaging seems to be warranted.
Biopsy
Biopsies of the primary tumor often can be performed in an outpatient setting.
Punch or cup forceps biopsy. This procedure is important in the diagnosis of mucosal lesions. The biopsy should be obtained at the border of the lesion, away from areas of obvious necrosis.
Fine-needle aspiration. Fine-needle aspiration (FNA) is a useful diagnostic modality. Multiple passes are made through the lesion with a fine-gauge (22-gauge) needle while suction is applied. Suction should be released before withdrawing the needle through surrounding soft tissue of the neck. FNA has an associated false-negative rate as low as 7%. The diagnostic accuracy depends on the physician’s skill and the cytopathologist’s experience.
Cytology is particularly useful in distinguishing a metastatic squamous cell carcinoma from other malignant histologies. However, a negative result should not be interpreted as “absence of malignancy.”
Core biopsy should not be performed on a neck mass, with the rare exception of a proven lymphoma.
Open biopsy. Open biopsy should be performed only when a diagnosis has not been made after extensive clinical evaluation and FNA is nondiagnostic. The operation should be performed only by a surgeon prepared to conduct immediate definitive surgical treatment at that time (which may entail a true neck dissection).
Pathology
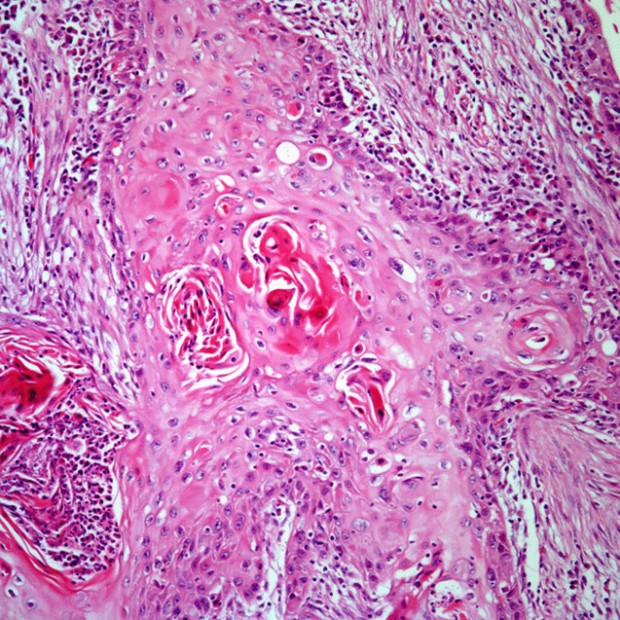
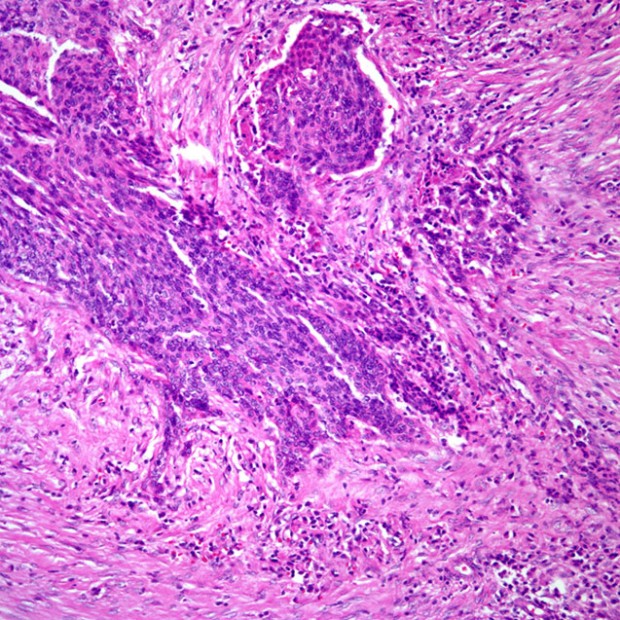
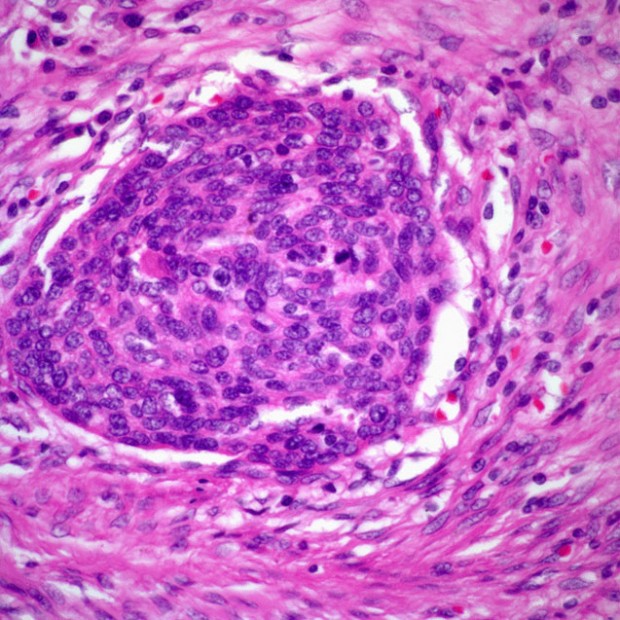
Squamous Cell Carcinoma
More than 90% of all head and neck cancers are squamous cell carcinomas.
Histologic grade
There are three histologic grades based on the amount of keratinization: A well-differentiated tumor is characterized by > 75% keratinization; a moderately differentiated tumor, by 25% to 50%; and a poorly differentiated tumor, by < 25%.
Histologic grade has not been a consistent predictor of clinical behavior. Features that predict aggressive behavior include perineural spread, lymphatic invasion, and tumor spread beyond the lymph node capsule. HPV-positive tumors tend to be non-keratinizing and poorly differentiated.
Morphologic growth patterns
Four morphologically distinct growth patterns have been recognized. The ulcerative type, the most common form, begins as a round or oval ulcer that is friable. Ulcerative lesions progress toward infiltration. Infiltrative lesions extend deeply into underlying tissues. The exophytic type tends to grow more superficially and metastasize later than the other types. It begins as an area of thickened epithelium.
Verrucous cancer is an uncommon variant that, in the United States, typically occurs in elderly patients with poor oral hygiene or ill-fitting dentures. It is characterized by a warty, bulky, elevated, fungating appearance. Verrucous cancers seldom metastasize.
Molecular markers
The inactivation of TP53, a central event in head and neck carcinogenesis, occurs by means of several prognostically significant mechanisms. Over 50% of head and neck squamous cell carcinomas have been found to harbor a mutation of the TP53 gene, and these are linked to diminished survival. HPV, which causes inactivation of TP53 via elaboration of E6 and E7 oncogenes, has been identified in 35% to 75% of oropharyngeal squamous cell carcinomas and has been associated with an improved clinical outcome.
In 2007, a case-control study providing epidemiologic evidence of a causal role for HPV in oropharyngeal cancer was published. Oral infection with HPV-16 or any of the other 37 HPV subtypes was independently associated with oropharyngeal cancer. HPV-16 was identified in 72% of oropharyngeal tumors. Patients without a history of smoking and/or ethanol abuse were more likely to harbor oral HPV-16. The tumor-suppressor p16 has been identified as a surrogate biomarker for HPV-positive oropharyngeal squamous cell carcinoma. In one series of 79 oropharyngeal tumors, p16 expression was measured by quantitative immunohistochemistry and HPV-16 infection was assayed by polymerase chain reaction. In fact, patients who had tumors that were HPV-positive and p16-positive had an improved survival compared with HPV-negative/p16-negative and HPV-positive/p16-negative patients.
Interestingly, the prevalence of p16-positive tumors is increasing. In successive Radiation Therapy Oncology Group (RTOG) studies, the prevalence has increased from 39.5% (RTOG 90-03, which accrued from 1991-1997) to 68% (RTOG 01-29, which accrued from 2002-2005). In RTOG 90-03, the risk of death doubled (hazard ratio [HR], 2.19; 95% CI, 1.46 to 3.28) among those who smoked during radiotherapy, after accounting for pack-years and other factors, and risk of second primary tumors increased by 1.5% per pack-year (HR, 1.015; 95% CI, 1.005 to 1.026).
Other Tumor Types
Other, less common head and neck cancers include mucoepidermoid carcinoma, adenoid cystic carcinoma, and adenocarcinoma, all of which may arise in the salivary glands. Head and neck cancers with neuroendocrine features include small-cell undifferentiated cancer and esthesioneuroblastoma (olfactory neuroblastoma). Both Hodgkin lymphoma and non-Hodgkin lymphoma may also be diagnosed as head and neck tumors, often involving the lymph nodes of the neck or Waldeyer ring.
Precancerous Lesions
There is a sequence of disease progression from atypia/dysplasia through carcinoma in situ to frankly invasive cancer. Leukoplakia and erythroplakia are terms applied to clinically identifiable lesions that may harbor invasive cancer or undergo malignant transformation.
Pathology
Leukoplakia results from chronic irritation of mucous membranes by carcinogens; this irritation stimulates the proliferation of white epithelial and connective tissue. Histopathologic examination reveals hyperkeratosis variably associated with underlying epithelial hyperplasia. In the absence of underlying dysplasia, leukoplakia rarely (< 5%) is associated with progression of disease to malignancy.
Erythroplakia
This is characterized by superficial, friable, red patches adjacent to normal mucosa. It is commonly associated with underlying epithelial dysplasia and has a much greater potential for malignancy than leukoplakia. Carcinoma is found in nearly 40% of erythroplakia cases.
Dysplasia
Dysplasia is characterized by cellular atypia, loss of normal maturation, and loss of normal epithelial stratification. It is graded as mild, moderate, or severe, based on the degree of nuclear abnormality present. In the transition from mild to severe dysplasia, nuclear abnormalities become more marked, mitoses become more apparent, and these changes involve increasing depth of epithelium. The likelihood of developing a carcinoma relates to the degree of dysplasia. In the case of severe dysplasia, as many as 24% of patients may develop invasive squamous cell cancer.
Carcinoma in situ
This is characterized by the presence of atypical changes throughout the epithelium, with complete loss of stratification. It is estimated that approximately 75% of invasive squamous cell carcinomas have an associated in situ component. Specific DNA mutations have also been identified in the sequence of disease progression from mild dysplasia to atypia to carcinoma in situ to invasive carcinoma.
Field Cancerization
Field cancerization is an important concept related to the natural history of head and neck cancer. This term describes the diffuse epithelial injury throughout the head and neck, lungs, and esophagus that results from chronic exposure to carcinogens.
Clinically, field cancerization is manifested by the frequent occurrence of (1) mucosal abnormalities, such as leukoplakia and dysplasia, beyond the margins of a head and neck cancer, and (2) second primary tumors within this exposed field. The lifetime risk of a patient with head and neck cancer developing a new cancer is 20% to 40%. Over time, as the risk of relapse of the initial cancer declines, the development of a new cancer represents the greatest risk for these patients.
Regional and Distant Metastases
The incidence of lymph node metastases is related to the size and thickness of the primary tumor. If the primary site is near the midline, contralateral or bilateral metastases should be anticipated. In the presence of lymph node metastases, extracapsular spread of tumor is an important prognostic factor.
Staging and Prognosis
Staging System
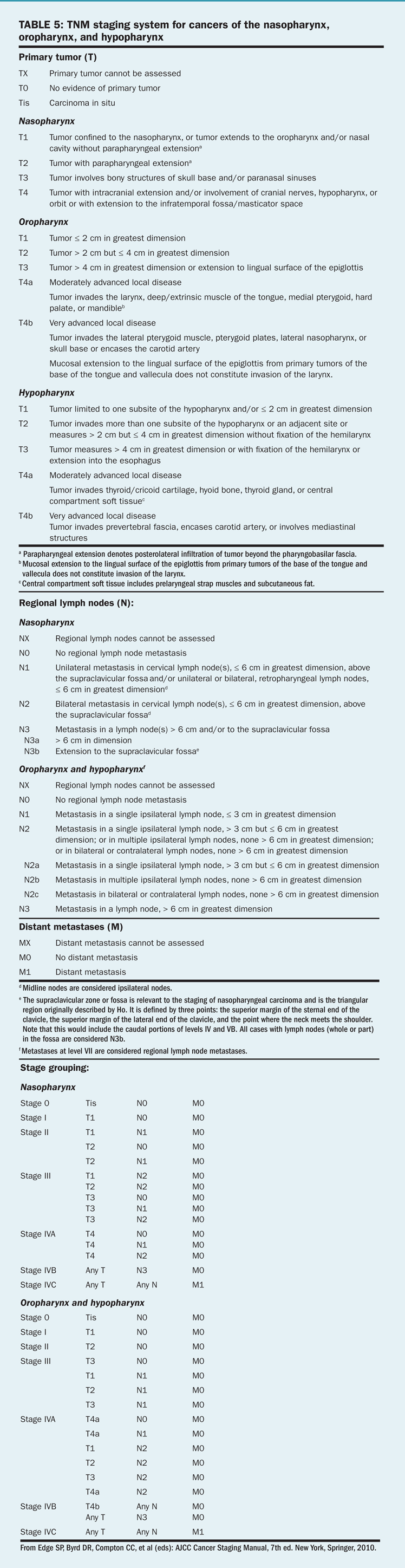
The TNM staging system of the American Joint Committee on Cancer (AJCC) maintains uniformity in the staging of head and neck tumors. The staging of primary mucosal tumors of the head and neck varies with the anatomic location and will be covered later by site. However, the staging systems for metastases and stage groupings are nearly uniform for all mucosal sites.
Prognosis correlates strongly with stage at diagnosis. For many head and neck cancer sites, survival of patients with stage I disease exceeds 80%. For patients with locally advanced disease at the time of diagnosis, (ie, stages III and IV disease), survival drops below 40%. Development of nodal metastases reduces survival of a patient with a small primary tumor by approximately 50%. Involvement of even a single lymph node is associated with a marked decline in survival. Most patients with head and neck cancer have stage III or IV disease at diagnosis.
Pattern of Relapse
Despite aggressive primary treatment, the majority of relapses that occur following a head and neck cancer are within the head and neck. Locoregional relapse accounts for approximately 80% of primary treatment failures. Distant metastases increase as the disease progresses and most often involve the lungs and, to a lesser extent, the bones and liver, which is why the addition of PET scans is of minimal utility in evaluating for distant cancer spread. By the time of death, 10% to 30% of patients will have clinically detected distant metastases.
Treatment Approaches
In general, early-stage (stage I or II) head and neck tumors may be treated using a single modality (surgery or radiotherapy), whereas advanced disease (stage III or IV) frequently benefits from multimodality therapy.
The best therapeutic approach for the primary tumor depends on the anatomic site. Most neck disease can be treated equally well with surgery or radiation, thus the modality chosen to treat the neck is based on which modality is selected for the primary. When the primary tumor is treated with irradiation, the “at-risk” regional lymphatics are incorporated into the treatment fields. Neck dissections should remain standardized (ie, complete anatomic dissections, as opposed to “berry picking” or random biopsy) in these settings to avoid incomplete surgery.
Preoperative Assessment
Before surgical resection, preoperative assessment of the extent of disease is essential. Complete physical examination and appropriate radiologic evaluation are necessary. Direct laryngoscopy and esophagoscopy are frequently performed to determine tumor extent and rule out the presence of a second primary tumor. A chest x-ray or CT scan may be obtained to screen for distant metastases or a primary lung cancer.
Surgical Principles
Classic principles of surgical oncology apply to head and neck cancer. Complete resection is necessary. Securing sufficient margins may be challenging because of the many structures in this area. Reconstruction is complex after resection of head and neck tumors, as the surgery may have an impact on appearance, speech, and swallowing. Decisions regarding the extent of resection should be made by experienced surgeons.
Surgery Plus Radiation Therapy
The combination of radical surgery and radiation therapy has been used for several decades to treat patients with advanced head and neck cancers.
Postoperative versus preoperative radiation therapy
Postoperative radiation therapy (60 to 70 Gy in 6 to 7 weeks) reduces the rate of locoregional recurrence from approximately 50% to 15% for tumors with pathologic features predictive of locoregional recurrence. The indications for postoperative radiation therapy are well established and include a deeply infiltrative primary tumor (T4); close or positive margins; an involved lymph node > 3 cm or multiple involved lymph nodes; extracapsular extension; an open lymph node biopsy not followed by an immediate neck dissection; perineural invasion; invasion of the lymphovascular space, cartilage, bone, or deep soft tissue; and surgeon unease (an experienced surgeon’s recommendation to deliver postoperative radiation should be credited by the radiation oncologist because the surgeon will often appreciate worrisome features not documented in a pathology report). The addition of postoperative radiation therapy reduces the risk of locoregional failure but does not decrease the risk of developing distant metastases.
Preoperative radiotherapy (45 to 50 Gy in 4 to 5 weeks) has been used for patients with advanced primary tumors, but rates of locoregional recurrence appear to be lower and complications fewer compared with postoperative radiation therapy; however, this observation was based on a study performed in the 1970s. Preoperative radiotherapy with or without chemotherapy is indicated for marginally resectable tumors, such as those with fixed cervical lymph nodes. In this setting, preoperative irradiation often permits resection of an otherwise unresectable tumor. However, this approach is used rarely because of the improvements in outcome with chemoradiation and the poor functional outcomes of patients requiring trimodality therapy.
Postoperative chemotherapy/radiation therapy
Two randomized clinical trials were launched to determine whether the addition of chemotherapy to radiation therapy enhanced locoregional tumor control and survival in high-risk patients with head and neck cancer following definitive surgical resection. The results of these trials were published in 2004. In the RTOG 95-01/Intergroup trial, patients with high-risk features including two or more involved lymph nodes, extracapsular extension, or positive margins following definitive surgical resection were randomized to receive 6,000 to 6,600 cGy of postoperative irradiation with or without cisplatin (100 mg/m2 given on days 1, 22, and 43). With a median follow-up of 45.9 months, the estimated 2-year rate of local and regional tumor control was 82% in the combined-therapy group vs 72% in the radiotherapy group (P = .01). The disease-free (P = .04), but not overall (P = .19), survival was significantly longer in the combined-treatment group, although the primary endpoint was locoregional control and was not powered to demonstrate a survival advantage. The incidence of acute adverse effects (grade 3 or greater) was higher in the combined-treatment group (77% vs 34%; P < .001).
In the European Organisation for Research on the Treatment of Cancer (EORTC) 22931 trial, similar conclusions were reported. This study included patients with T3 or T4 disease, perineural invasion, and involvement of the lymphovascular space in addition to positive margins and extracapsular extension. The overall survival rate was significantly improved in the combined-therapy group compared with the radiotherapy group. After a mean of 60 months, the overall survival of 334 randomized patients was 53% in the combined-treatment group vs 40% in the radiation-alone group (P = .02). The discrepancy in effect on overall survival between the two studies was thought to be related to different entry criteria used in the RTOG and EORTC trials.
In a comparative analysis of the two trials, the presence of extracapsular extension and/or microscopically involved surgical margins were the only risk factors for which the impact of chemoradiation therapy significantly improved survival.
Curative radiation therapy
Radiation therapy with curative intent usually involves daily treatment for 6 to 7 weeks (total dose: 60 to 70 Gy). Although there is no tissue loss with radiation therapy, as there is with surgery, potential complications include dry mouth, tissue fibrosis, trismus, bone necrosis, hypothyroidism, and dysphasia. Some problems are common and sufficiently debilitating that they require significant care during treatment planning. Surgery often produces less morbidity in the oral cavity, whereas radiotherapy produces less morbidity in the oropharyngeal, laryngeal, and nasopharyngeal regions.
Radiation fractionation
The RTOG 90-03 trial was conducted to determine the efficacy of various fractionation schemes in the treament of locally advanced head and neck cancer. Four schedules were tested: (1) standard fractionation at 2 Gy/fraction/d, 5 d/wk, to 70 Gy/35 fractions/7 wk; (2) hyperfractionation at 1.2 Gy/fraction, twice daily, 5 d/wk to 81.6 Gy/68 fractions/7 wk; (3) accelerated fractionation with split at 1.6 Gy/fraction, twice daily, 5 d/wk to 67.2 Gy/42 fractions/6 wk, including a 2-week rest after 38.4 Gy; or (4) accelerated fractionation with a concomitant boost at 1.8 Gy/fraction/d, 5 d/wk, and 1.5 Gy/fraction/d to a boost field as a second daily treatment for the last 12 treatment days to 72 Gy/42 fractions/6 wk. A total of 1,113 patients were entered in the study, with a median follow-up of 23 months.
Patients treated with both hyperfractionation and accelerated fractionation with a concomitant boost had significantly increased locoregional tumor control rates (the primary endpoint) on RTOG 90-03 compared with patients on the other two arms. All three groups treated with the altered fractionation schemes had more acute, but not late, side effects. The study concluded that hyperfractionation and accelerated fractionation with a concomitant boost were both superior to conventionally fractionated radiotherapy.
An update of this trial further demonstrated that the hyperfractionated arm showed a trend toward an overall survival benefit (P = .06); the accelerated fractionation arm using the concomitant boost showed a trend toward increased late side effects (P = .06).
A recent, large meta-analysis that analyzed different fractionation schedules demonstrated an 8% improvement in overall survival with hyperfractionation similar to the benefit of added concurrent chemotherapy seen in the 1995 meta-analysis.
Intensity-modulated radiation therapy
Intensity-modulated radiation therapy (IMRT) is a more sophisticated approach for obtaining highly conformal radiation dose distributions needed to irradiate complex targets positioned near sensitive normal structures. Treatment planning for IMRT (also known as inverse planning) is extremely complex and different from that of conventional or three-dimensional radiation therapy planning. The starting point with IMRT is a description of the desired dose distribution rather than the application of traditional fields and beam modifiers to generate an acceptable plan. Conventional radiation treatment utilizes relatively uniform beams of radiation (typically between 2 and 4 beams), whereas IMRT, instead of using 4 beams of 50 cGy each, could use 50 beams of 4 cGy each. Each beam direction is divided into multiple segments to modulate the radiation dose.
The role of IMRT continues to evolve, although its use should not be considered standard for all head and neck tumors. One randomized trial in patients with early nasopharyngeal cancer showed a small improvement in salivary function. IMRT in head and neck cancer is ideal in the setting of a tumor adjacent to a critical structure (eyes, optic nerve, spinal cord, brainstem, optic chiasm, or spinal cord), which would not otherwise be treated adequately with conventional planning.
Various groups have examined dosimetric and quality-of-life differences between IMRT and conventional radiation techniques. The group at Memorial Sloan-Kettering Cancer Center compared its IMRT planned treatment for nasopharyngeal cancer against conventional treatment with a conformal boost. Locoregional tumor control was 97% vs 78%, with IMRT vs conventional radiotherapy, respectively, at 2 years. The University of Michigan and Washington University have reported reductions in late xerostomia with IMRT.
Proper patient selection is imperative when using IMRT. Marginal failures have been reported in the postoperative setting, in which no consensus guidelines exist regarding optimal target volume, and in the setting of bulky adenopathy. The postoperative setting is difficult, because disruption of normal draining lymphatics makes the appropriate target volume much more difficult to define. Bulky adenopathy may change the typical drainage pattern and lead to metastases in the base of the skull or parotid glands.
In addition, the RTOG-0234 group reported that IMRT in the postoperative setting increased acute side effects in high-risk patients when compared with three-dimensional conformal radiotherapy. This increase in side effects may be related to dosimetric parameters such as “hot spots” or a greater volume of normal structures receiving low doses.
In the definitive setting, all series have demonstrated improved outcomes. RTOG H-0022 was a multi-institutional study using IMRT for T1 or T2, N0 or N1 squamous cell carcinoma of the oropharynx to 66 Gy in 30 fractions. Investigators found that moderately accelerated hypofractionated IMRT without chemotherapy for early oropharyngeal cancer is feasible, achieving high tumor control rates (95% local control) and reduced salivary toxicity when compared with treatment of similar patients in previous RTOG studies.
Chemotherapy
Induction (neoadjuvant) chemotherapy vs concomitant chemotherapy and radiation therapy. A rationale for using induction chemotherapy for treating advanced-stage laryngeal cancer involves using chemotherapy as a marker of radiation sensitivity to select potentially radiocurable patients. In the Veterans Administration (VA) Laryngeal Cancer Cooperative Study, the major benefit for two-thirds of patients in the experimental arm was laryngeal preservation. In this study, the lack of a substantial tumor response to induction chemotherapy was not associated with reduced survival. Patients who failed to respond to induction chemotherapy underwent surgery, which had the advantage of not being performed in an irradiated field. One problem with this approach is that radiation sensitivity may not be related to chemotherapy. For example, in the RTOG 91-11 trial, 11 of 12 patients who refused laryngectomy because of lack of a response to chemotherapy were nevertheless salvaged with radiotherapy, suggesting that response to induction chemotherapy may not predict radioresponsiveness.
The use of concurrent treatment (as opposed to induction) using chemotherapy and irradiation is associated with increased locoregional tumor control and survival. When used concurrently with radiotherapy, the function of chemotherapy is thought to “radiosensitize” the tissue in the radiation field. The RTOG 91-11 trial demonstrated improved locoregional tumor control as well as an increased rate of laryngeal preservation in patients with stage III and IV resectable laryngeal cancer who underwent concurrent chemoradiation therapy (for details, see discussion later in chapter). However, the rate of mucosal toxicity in those receiving concomitant chemotherapy was double that of either of the other two arms. Patients with extensive T4 primaries, such as those with cartilage invasion or those with involvement of the tongue base, were not included in the study because of poor outcomes in the VA larynx study.
Investigators from the University of Michigan also studied the role of induction chemotherapy in advanced laryngeal cancer and conducted a phase II trial in which patients who had a response of greater than 50% to a single cycle of induction cisplatin (100 mg/m2) and 5-fluorouracil (5-FU) (1,000 mg/m2/d, days 1 to 5) went on to receive definitive chemoradiation. Of the 97 patients, 33% had T4 primary tumors. Seventy-three patients (75%) were able to undergo chemoradiation, and the overall laryngeal preservation rate was 70%. After a median follow-up of 41.9 months, the overall 3-year survival rate was 85%.
Biologic agents. The toxicity of chemotherapeutic agents has prompted the development of molecular agents with potentially greater tumor specificity and diminished toxicity. Targeting the epidermal growth factor receptor has thus been a subject of study in squamous cell head and neck cancers. In one study, 424 patients with locoregionally advanced head and neck cancer were randomly assigned to receive treatment with high-dose radiotherapy alone or with weekly cetuximab (Erbitux). The primary endpoint was locoregional control; secondary endpoints included overall and progression-free survival. The median duration of locoregional tumor control was 24.4 months among patients treated with cetuximab plus radiotherapy and 14.9 months among those given radiation therapy alone (HR for locoregional progression or death, 0.68; P = .005). With a median follow-up of 54 months, the median duration of overall survival was 49 months among patients treated with combined therapy and 29.3 months among those treated with radiation therapy alone (HR for death, 0.74; P = .03). Radiotherapy plus cetuximab significantly prolonged progression-free survival (HR for disease progression or death, 0.70; P = .006) In March 2006, cetuximab received US Food and Drug Administration (FDA) approval for use in combination with radiotherapy for patients with squamous cell cancer of the head and neck as well as for monotherapy metastatic disease. Combined-modality treatment was not associated with any increased mucosal toxicity or long-term differences in measured quality of life. Other agents are being investigated.
The cooperative groups are evaluating the benefit of cetuximab and conventional chemotherapy with radiation in the locally advanced and high-risk postoperative setting, since the added effects from cetuximab are low and not overlapping with cytotoxic therapy. The initial findings from RTOG 05-22 presented at the 2011 Annual Meeting of the American Society of Clinical Oncology (ASCO) did not demonstrate an improvement (median follow-up, 2.4 years) with the addition of cetuximab to cisplatin-based concurrent chemoradiotherapy, although longer follow-up and further analysis based on HPV status are still needed to confirm these findings.
Chemotherapy and survival. Numerous clinical trials have shown an improvement in locoregional tumor control using concurrent chemoradiation therapy, but a survival benefit has been observed less consistently. Recently published information from an updated meta-analysis of chemotherapy in head and neck cancer reports individual patient outcomes for 50 trials of concomitant chemoradiation therapy compared with irradiation alone. The pooled HR was 0.81, with a P < .001 and an absolute survival benefit of 8% at 5 years for concomitant treatment. In contrast, neoadjuvant chemotherapy with cisplatin and 5-FU prior to radiation did not improve survival, demonstrating the importance of timing with respect to the integration of chemotherapy in primary management.
In the RTOG 91-11 randomized trial, chemotherapy given concurrently with irradiation, or sequentially prior to irradiation, suppressed the incidence of distant metastases and improved disease-specific survival relative to irradiation alone. However, overall survival was not different among all three arms.
Concurrent chemoradiation therapy has the greatest impact on survival in the setting of unresectable squamous cell cancer, based on a head and neck Intergroup study. In this study, 295 patients with stages III and IV head and neck cancer were randomized to participate in one of three arms: (A) radiotherapy alone to 70 Gy in 35 fractions; (B) 70 Gy in 35 fractions plus concurrent cisplatin on days 1, 22, and 43; and (C) split-course radiotherapy and three cycles or concurrent cisplatin/5-FU chemotherapy, with 30 Gy given with cycle 1 and 30 to 40 Gy given with cycle 3. Grade 3 or worse toxicity occurred in 53% of arm A patients, 86% of arm B patients, and 77% of arm C patients. The 2- and 3-year Kaplan-Meier projected survivals for arm A are 30% and 20%, compared with 43% and 37% for arm B (P = .016) and 40% and 27% for arm C (P = .13). Median survival was 12.6 months for arm A, 19.1 months for arm B, and 14 months for arm C. The addition of concurrent, high-dose, single-agent cisplatin to conventional radiotherapy significantly improved survival with acceptable toxicity. In addition, concurrent multiagent chemotherapy did not offset the loss of efficacy resulting from split-course irradiation.
The impact of adjuvant concomitant chemotherapy and radiotherapy on survival was assessed in two similarly designed trials in high-risk, postoperative head and neck cancer patients that tested adjuvant chemoradiation therapy against standard postoperative radiotherapy. In the RTOG 95-01 trial, disease-free survival was significantly increased in patients treated with adjuvant chemoradiation therapy relative to those treated with radiotherapy alone, but overall survival was not.
Multiple investigators have presented data on the ability of the excision repair cross-complementation group 1 (ERCC-1) enzyme to predict response in patients treated for head and neck cancer. ERCC-1 appears to be related to resistance to platinum-based chemotherapy among other cancer sites. In one retrospective study, patients who had low levels of ERCC-1 expression had four times greater odds of benefiting from an objective response to chemotherapy (P = .01), which corresponded to a lower risk of cancer-related death. A prospective study to evaluate the role of ERCC-1 as a predictive biomarker in head and neck cancer has yet to be conducted.
Induction chemotherapy in combination with concomitant chemoradiation therapy. Concurrent chemoradiation therapy is the current standard of care for patients with locally advanced squamous cell carcinoma of the head and neck. Many think this paradigm shift has influenced patterns of failure, and distant metastasis has become more important. Docetaxel (Taxotere) in combination with cisplatin and 5-FU (TPF) was approved by the FDA in 2007 for use as induction therapy for patients with locally advanced squamous cell cancer of the head and neck.
The trial that led to this indication was a multicenter, open-label, randomized, phase III evaluation of TPF compared with cisplatin and 5-FU (PF) for three cycles prior to chemoradiation with weekly carboplatin. A total of 501 patients with stage III or IV disease, deemed either unresectable or candidates for organ preservation, were randomized. Locoregional control was improved in the TPF arm (70% vs 62%). This translated into an improvement in overall survival, with median survival rates of 71 vs 30 months and an HR for survival in the TPF arm of 0.70 (P = .006). Distant failure was similar in both arms and was uncommon, observed in less than 10% of patients. Neutropenia and neutropenic fever were more common in the TPF group, but with the addition of prophylactic antibiotics, the regimen was feasible. Patient selection for this treatment approach is critical, given the toxicities and increased length of total therapy. In 2011, researchers reported long-term follow-up of patients receiving TPF vs PF, the historic standard induction therapy, showing that the trial results endured with time. With a median follow-up of 72.2 months, the median survival was 70.6 months in the TPF arm vs 34.8 months in the PF arm.
To date, however, there have been no data suggesting that this combination is better than concurrent chemoradiation alone. Physicians who oppose this approach are concerned about the risk of tumor progression on chemotherapy (10%) and, ultimately, the risk of patients not being able to initiate and/or complete the definitive chemoradiation component of this treatment. Two randomized trials evaluating these two approaches were reported at ASCO 2012. Unfortunately, the PARADIGM trial closed early secondary to poor accrual, with only 145 of the 300 planned patients. The induction regimen consisted of three cycles of cisplatin (100 mg/m2), docetaxel (75 mg/m2), and 5-FU (1000 mg/m2 for 4 days). This was followed by radiation with concurrent weekly carboplatin or weekly docetaxel among the nonresponders to induction. The control arm of chemoradiation used a 6-week course of accelerated boost radiation with two cycles of cisplatin (100 mg/m2). With a median follow-up of 49 months, there was no clear difference in progression-free or overall survival. The DeCIDE trial similarly accrued slowly and was amended to reduce the planned sample size, but the trial was eventually completed. This trial, which was led by the University of Chicago, used an accelerated hyperfractionated approach with planned split course (150 cGy twice daily for 5 days followed by a week break) to a total dose of 75 Gy as the standard radiotherapy arm given with concurrent docetaxel, hydroxyurea, and 5-FU. The induction arm included two cycles of docetaxel (75 mg/m2), cisplatin (75 mg/m2) and 5-FU (750 mg/m2, days 1 to 5). The use of induction therapy increased the incidence of grade 3 neutropenia and leukopenia but did not seem to impare the ability for patients to tolerate the full course of radiotherapy. The primary endpoint was overall survival, which based on the 280 subjects accrued provided 80% power to detect a, HR of 0.5. The 3-year outcomes demonstrated a decrease in distant metastases (19% to 10% favoring the induction arm; HR 0.46; P = .025), but this did translate into an overall survival advantage (75% vs 73% favoring the induction arm; HR 0.92; P = .70). Neither of these trials had prospectively assayed for HPV status among the oropharyngeal cancers, and thus this was not a factor for stratification in either study.
Adjuvant chemotherapy following surgery or irradiation. Adjuvant chemotherapy has been given following initial surgery or radiation therapy to eliminate microscopic residual disease and distant metastases. Although this approach has resulted in a reduced rate of distant metastasis, it has not been associated with improved locoregional tumor control or survival, except in a large meta-analysis. As concomitant approaches evolve and locoregional tumor control for advanced disease becomes the rule rather than the exception, the value of additional chemotherapy (as induction or adjuvant therapy) will need to be re-explored to address the problem of distant metastasis. Currently, there is interest in studying the role of targeted therapy in the adjuvant setting after definitive radiation, but this remains a research question.
Locally advanced head and neck cancer. Data from prospective trials continue to support the use of altered fractionation and concurrent chemotherapy and irradiation as an alternative to surgery or conventionally fractionated irradiation alone for locally advanced cancers of the head and neck. The long-term results of RTOG 90-03, which randomized 1,113 patients with stages III and IV squamous cell carcinoma of the oral cavity, oropharynx, or supraglottic larynx and stages II to IV squamous cell carcinoma of the base of the tongue or hypopharynx to receive one of four different schedules of irradiation alone, revealed that locoregional tumor control and disease-free survival were superior for the hyperfractionated arms and accelerated fractionation with the concomitant boost, compared with the conventional fractionated radiotherapy arm. The hyperfractionated arm demonstrated a trend toward improved overall survival.
Updated results for RTOG 99-14, a phase II prospective study designed to integrate the altered fractionated radiation regimen using concomitant boosts from RTOG 90-03 with chemotherapy, were also reported in 2005. Cisplatin was given during weeks 1, 3, and 5. Longer-term results for 76 of 84 patients were reported, and the 3-year disease-free, overall, and cause-specific survival rates were 45.8%, 57.7%, and 61.2%, respectively. The 3-year local recurrence and distant metastasis rates were 38.7% and 23.3%, respectively. Of the 35 patients who were alive, 22 had a temporary feeding tube and 17% (6 patients) still required a feeding tube at the time of their last follow-up.
RTOG 97-03, a three-arm randomized phase II trial, evaluated the feasibility of concurrent multi-agent chemotherapy with radiation therapy. In this study, three different regimens of chemotherapy were used with irradiation (70 Gy/35 fractions) for oral cavity, oropharyngeal, and hypopharyngeal cancers. Arm 1 received cisplatin (10 mg/m2) and 5-FU (400 mg/m2) via continuous infusion daily for the final 10 days of radiotherapy. Treatment in arm 2 consisted of hydroxyurea (1 g/d) and 5-FU (800 mg/m2) via continuous infusion delivered concurrently with radiation therapy. Arm 3 received paclitaxel (30 mg/m2) and cisplatin (20 mg/m2) weekly. The incidence of acute toxicity was high, with the lowest incidence of persistent feeding tube dependence in arm 3. Arm 2 was associated with a slightly higher rate of grade 4 toxicity and treatment interruption. All arms demonstrated improved survival relative to historic controls treated with radiation therapy alone.
To diminish toxicity in locally advanced oropharyngeal and laryngeal cancers, a phase II trial using taxane-based induction followed by concurrent chemoradiation therapy was conducted through the Eastern Cooperative Oncology Group (ECOG) 2399 group. A total of 111 patients (T2, N+ or T3–4N0–3) underwent induction therapy with two cycles of paclitaxel (175 mg/m2) and carboplatin followed by concurrent weekly paclitaxel (30 mg/m2) and 70 Gy of radiotherapy. Patients with progressive or stable disease at the primary site after induction therapy underwent surgery. Patients with primary laryngeal cancers fared significantly worse using this regimen. Patients with laryngeal cancer had a lower rate of major response to induction chemotherapy, a lower 2-year progression-free survival rate, and a trend toward higher distant failure than did those with primary oropharyngeal cancer. The 2-year progression-free survival was 50% for patients with laryngeal cancer, compared with 75% for those with oropharyngeal cancer (P = .05). The improved survival among patients with oropharyngeal cancer may be due to the increased number of HPV-positive patients in this subset. The presence of genomic HPV-16, HPV-33, and HPV-35 DNA was assayed on tumor samples of patients in this study. HPV-positive tumors had a higher response rate to induction therapy (82% vs 55%) as well as to chemoradiation (84 % vs 57%), compared with HPV-negative tumors. In addition, patients with HPV-positive tumors had an improved 2-year overall survival of 95%.
Given the known differences in biology and prognosis between HPV-positive and HPV-negative tumors, current trials for locally advanced oropharyngeal cancers are studying these two groups separately. In particular, the cooperative groups are interested in whether it is possible to de-intensify treatment for HPV-positive disease, with the eventual goal of minimizing late toxicites. In ECOG 1308, patients with HPV-positive tumors underwent induction chemotherapy with cisplatin, paclitaxel, and cetuximab. Those who had a complete response in the primary site received a lower dose of definitive radiation with cetuximab. This trial has completed accrual, but results have not been reported to date. The RTOG 1016 trial, which is currently ongoing, randomizes patients to either cetuximab or cisplatin with concurrent radiation.
Determination of tumor response after chemoradiation. For patients with persistent disease after radiation, prompt diagnosis and treatment may improve survival. However, the sensitivity of the physical examination is poor as a result of the presence of submucosal disease and inflammatory changes in the upper aerodigestive tract from radiation. The best method for assessing tumor response to radiation remains under investigation. The sensitivity of CT imaging in the early postradiotherapy period (ie, within 10 weeks of the completion of radiation) is 80% for the primary site and 87% for the regional lymphatics. This modality, however, lacks specificity. FDG-PET/CT imaging has been shown to improve the positive predictive value of CT scans in determining response to radiation. A prospective study of 98 patients who underwent radiation for head and neck cancer did not support the use of FDG-PET/CT rather than CT imaging alone in evaluating treatment response early after the completion of radiotherapy. Overall, CT imaging is more sensitive when used during the early postradiation period. However, for high-risk patients (eg, current or former smokers, those with HPV-negative and non-oropharyngeal cancers), PET/CT scans enhanced the positive predictive value when compared with CT scan alone (100% in the primary site and 75% in the cervical lymph nodes compared with 71% and 37%, respectively, for CT imaging). This suggested that early PET/CT scanning may accurately identify patients at high risk for failure who may benefit from salvage surgery.
Chemotherapy for recurrent or metastatic disease. The combination of cisplatin/5-FU produces overall response rates of approximately 30% and survival rates of 6 months. In randomized trials comparing this combination with single-agent cisplatin, 5-FU, or methotrexate, response rates with the single agents are lower, but survival is equivalent. However, because many practitioners believe response is a surrogate for palliation, cisplatin/5-FU has been used widely in this setting.
The taxanes are the most active cytotoxins yet identified in head and neck cancer, with overall response rates of approximately 35% in patients with recurrent or incurable disease. In one randomized trial, the combination of paclitaxel/cisplatin was compared with cisplatin/5-FU in patients with recurrent disease. A total of 194 patients who were therapy-naive for recurrent disease with an ECOG performance status of 0–1 were randomized. Efficacy outcomes were similar in the two arms, with median survival rates of 8 and 9 months for paclitaxel/cisplatin compared with cisplatin/5-FU, respectively. Paclitaxel/cisplatin was less toxic and more convenient, representing an effective and safe alternative to the traditional 5-FU–containing regimen.
Based on nearly universal expression of EGFR in squamous cell cancer of the head and neck, EGFR-targeted agents have been evaluated in patients with recurrent disease. Although the single-agent response rates of the monoclonal antibody cetuximab and the tyrosine kinase inhibitors erlotinib (Tarceva) and gefitinib (Iressa) in this setting are only in the range of 5% to 15%, their use in combination with chemotherapy, particularly cetuximab, holds more promise. Results of the EXTREME (Erbitux in First-Line Treatment of Recurrent or Metastatic Head and Neck Cancer) trial, which accrued 442 patients with recurrent or metastatic squamous cell cancer of the head and neck, showed that when compared with platinum-based chemotherapy alone, the combination with cetuximab improved median (7.4 months vs 10.1 months) and 1-year (31% vs 39%) survival rates, with an HR of 0.8 (P = .036). Similar promising, albeit preliminary, results have been reported from phase II, single-arm studies of cetuximab/paclitaxel and erlotinib/docetaxel/cisplatin. Overall, these observations lend impetus to further investigation of EGFR-targeted therapy in combination with neoadjuvant chemotherapy and with chemoradiation therapy in patients with curable disease.
Other Therapies
Photodynamic therapy
Photodynamic therapy may have some promise in the treatment of mucosal dysplasia and small head and neck tumors.
Small studies of photodynamic therapy, performed at several institutions, suggest that widespread areas of carcinoma in situ or severe dysplasia, as well as cancer, are often extirpated after photodynamic therapy. Some patients have experienced durable remissions, but the long-term efficacy of this modality remains uncertain.
Pulsed dye laser therapy for early laryngeal lesions
The pulsed dye laser initially used to treat vascular lesions has recently been investigated in the treatment of respiratory papillomatosis, dysplastic cancers, and early invasive glottic carcinomas. The efficacy of the laser is mediated through its antiangiogenic properties. The nontoxic treatment preserves the laryngeal microarchitecture and preserves or restores near-normal vocal quality. In addition, this modality can be used in an outpatient clinic setting. Preliminary findings are favorable. At this time, however, pulsed dye laser therapy should be considered investigational.
Chemoprevention
The area of chemoprevention has received a great deal of attention in recent years, and the concept of field cancerization is important in this context. As mentioned previously, this concept refers to the diffuse epithelial injury incurred by upper aerodigestive tract mucosa due to chronic exposure to carcinogens (most commonly, alcohol and tobacco). These mucosal changes increase the risk of developing premalignant lesions (leukoplakia and erythroplakia) as well as multiple primary lesions.
Retinoids (vitamin A analogues) have been investigated as chemopreventive agents in the aerodigestive tract based on their efficacy in tumor models, such as the hamster buccal pouch. Retinoids mediate changes in gene expression through interaction with nuclear retinoic acid receptors, which function as transcription factors. Nutritional epidemiology studies also have indicated that low serum levels of both carotenoids and retinoids contribute to the risk of cancer development in the epithelium of the upper aerodigestive tract.
The role of retinoids in the prevention of second primary tumors in patients treated with curative intent for an early-stage index squamous cell cancer of the head and neck has been investigated in three randomized trials. The initial study was a small, single-center, phase III trial of cis-retinoic acid (1 to 2 mg/kg/d) by Hong et al. Although this study demonstrated decreased second primary tumors in the experimental arm, compliance and morbidity with this high dose were problematic. A second study using a low dose of cis-retinoic acid (10 mg/d) was performed by ECOG, and results were negative. A third, large, multicenter trial with an intermediate dose of 30 mg/d has also reported negative results in regard to second primary prevention.
Rehabilitation
Rehabilitation also is very important in the care of patients with head and neck cancer. It includes physical and occupational therapy, speech and swallowing rehabilitation, and nutritional support. For example, resection of the spinal accessory nerve, which innervates the trapezius muscle, leads to scapular winging, an inability to abduct the arm fully and, eventually, severe pain around the shoulder. These symptoms may be ameliorated with appropriate physical therapy.
Adapting to the loss of the larynx also requires intensive rehabilitation and patient motivation. Voice rehabilitation options include esophageal speech, artificial larynges (portable, battery-operated devices), and tracheoesophageal shunts.
Patients who undergo nonsurgical treatment also benefit from early swallowing rehabilitation. Intensive chemoradiation protocols are associated with prolonged feeding tube dependence in 20% of patients. Early swallowing therapy improves treatment-related dysphagia.
Nutritional support is facilitated by temporary nasoduodenal tubes or gastrostomy tubes (which impose added morbidity but are more socially acceptable and ease the patient’s transition to normal activities).
Management of Symptoms and Treatment Side Effects
Photodynamic therapy. At the time of diagnosis, many patients with head and neck cancer will have lost a significant amount of weight. Maintaining adequate nutrition is a major problem for these patients, as both the tumor and treatment side effects, such as mucositis from chemotherapy and radiation therapy, may be contributory. For patients who are unable to eat or who are being treated with aggressive concomitant chemotherapy and radiation therapy protocols, placement of a gastrostomy tube is often necessary to maintain caloric intake and adequate hydration.
Pain. Clinicians must also be aware of the significant pain associated with these lesions and use narcotic analgesics appropriately to relieve discomfort.
Mucositis. The use of chemotherapy concomitantly with radiation therapy increases the occurrence of mucositis.
Nephrotoxicity and ototoxicity. For patients treated with cisplatin-containing regimens, renal insufficiency and ototoxicity are potential serious side effects.
Xerostomia. Following radiation therapy of a head and neck cancer, xerostomia may be a significant long-term side effect. In some patients, pilocarpine has been useful in stimulating the production of saliva.
The use of organic thiophosphates, such as amifostine (Ethyol), in patients undergoing radiotherapy for head and neck cancer reduces the severity of late xerostomia without compromising the antitumor activity of the irradiation.
Gastroesophageal reflux disease (GERD). Often asymptomatic or “silent,” GERD is a common finding in patients treated for pharyngolaryngeal squamous cell carcinoma. In addition, cisplatin-containing chemotherapy may aggravate GERD.
Treatment of the Neck
Either irradiation alone or radical neck dissection will control metastatic squamous cell cancer to a single small neck node more than 90% of the time if there is no extracapsular tumor spread. Hence, radiation treatment may easily provide prophylactic treatment of the neck if control of the primary tumor is undertaken with irradiation. Traditionally, if the tumor in the neck was N2 or greater, or if there was tumor beyond the confines of a node, neck dissection and irradiation were combined for optimal control of the neck tumor. The need for neck dissection in patients with clinically staged N2–N3 tumors with a complete clinical and radiographic response following radiation therapy remains under investigation.
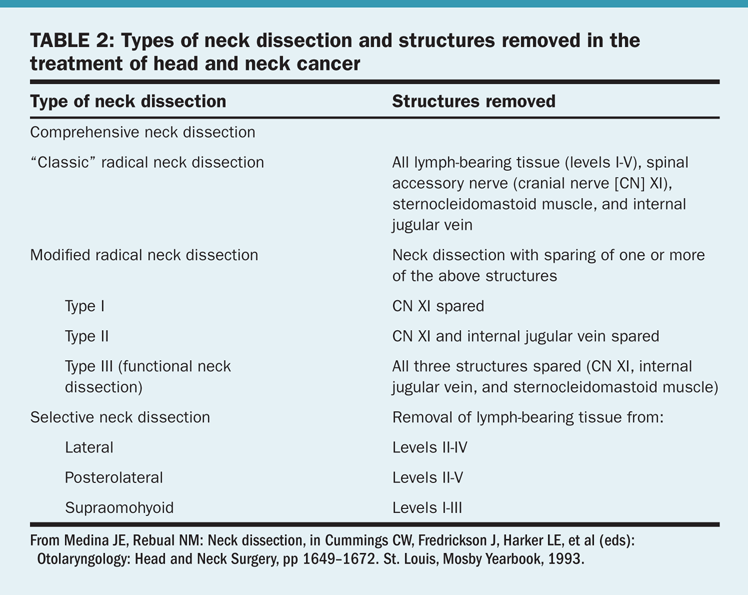
Types of dissection. There are several approaches to the surgical treatment of the neck nodes in patients with head and neck cancer (Table 2). This discussion will be limited to two types of neck dissection: comprehensive and selective.
• Comprehensive neck dissection—This entails complete removal of all lymphatic tissue from the neck (levels I–V). A radical neck dissection includes comprehensive node dissection with removal of the sternocleidomastoid muscle, jugular vein, and spinal accessory nerve. Modified radical neck dissection was developed to diminish the morbidity of the classic operation. The most important structure to preserve is the spinal accessory nerve.
• Selective neck dissection—This consists of the removal of lymph node groups at highest risk for containing metastases from a primary cancer. In such procedures, the lymph nodes removed correspond to the most significant drainage basins of specific head and neck tumor sites. These are staging operations usually performed in patients with clinically N0 neck cancer. If metastases are identified, further treatment to the neck will be required. A selective neck dissection should not be employed as the sole treatment of clinically palpable disease.
Sentinel lymph node biopsy for oral cavity lesions has been evaluated. Forty patients with clinically N0 neck cancer underwent sentinel lymph node biopsy followed by complete neck dissection. A sentinel node was identified in 90% of necks, with a 97% accuracy rate in predicting the nodal status of the remainder of the neck. This finding corresponded to a sensitivity of 94% and a specificity of 100%. Although these results are encouraging, they need to be validated in a larger trial. The completed American College of Surgeons Oncology Group (ACOSOG) study (Z0360) examined this technique in patients with T1 or T2, N0 oral cavity cancer. The final conclusions are pending. While sentinel lymph node biopsy may prove useful in small lesions without deep penetration, it remains investigational.
Follow-Up of Long-Term Survivors
As mentioned, head and neck cancers are aggressive tumors. The majority (80%) of recurrences will develop within 2 years. Since many recurrences are treatable with curative intent, patients should be followed closely during the months following their treatment. This period coincides with the time of greatest need from the standpoint of rehabilitation.
After 2 years, second primary tumors of the head and neck and lungs become important causes of death and morbidity. Late complications of treatment, such as radionecrosis, dental decay, radiation-induced fibrosis, hypothyroidism, and sequelae of spinal accessory nerve sacrifice or injury, may develop even after years. Complications and second primary cancers are more common in patients who continue to smoke.
Timing of Follow-Up Evaluations
Follow-up evaluations at regular intervals should be complete and should include a focused history and examination, as outlined previously. Physicians who are able to perform a head and neck examination (including laryngoscopy) should direct follow-up. After surgical treatment, this evaluation will usually require visits with the head and neck surgeon. Patients treated with irradiation should be followed by both their radiation oncologist and a head and neck surgeon or otolaryngologist.
Evaluations should be scheduled every 1 to 2 months during the first year after treatment, every 2 to 4 months during the second year, every 3 to 6 months during the third year, and every 6 months thereafter for several more years.
Imaging and Laboratory Studies
Any mucosal abnormality should be biopsied. There are no tumor markers or other useful laboratory studies to follow. Chest x-rays should be obtained yearly. There is little justification for performing CT or MRI in the follow-up of asymptomatic patients. Thyroid-stimulating hormone (TSH) should be measured yearly in patients.
Head and Neck Tumor Regions
As mentioned previously, tumors occurring at different anatomic sites and subsites of the head and neck vary considerably with regard to epidemiology, risk factors, anatomy, natural history, staging of the primary tumor, and therapy. The following sections highlight these differences.
Oral Cavity
Sites of the oral cavity include the lips, hard palate, floor of the mouth, buccal mucosa, and tongue. Cancers at these sites account for less than 5% of all malignancies in the United States.
Anatomy
The oral cavity extends from the cutaneous vermilion junction of the lips to the junction of the hard and soft palates above and to the line of the circumvallate papillae below. It includes the lips, buccal mucosa, upper and lower alveolar ridges, retromolar trigone, floor of the mouth, hard palate, and anterior two-thirds of the tongue (the “oral” tongue). The primary lymphatic drainage is to the submental triangle, submandibular nodes, and upper deep jugular nodes.
Natural history
The most common presenting complaint is a sore in the mouth or on the lips. One-third of patients present with a neck mass.
The differential diagnosis includes other malignancies and benign diseases or lesions. Other malignancies to be considered include salivary gland tumors, sarcoma, lymphoma, and melanoma. Benign diseases include pyogenic granuloma, tuberculous disease, aphthous ulcers, and chancres.
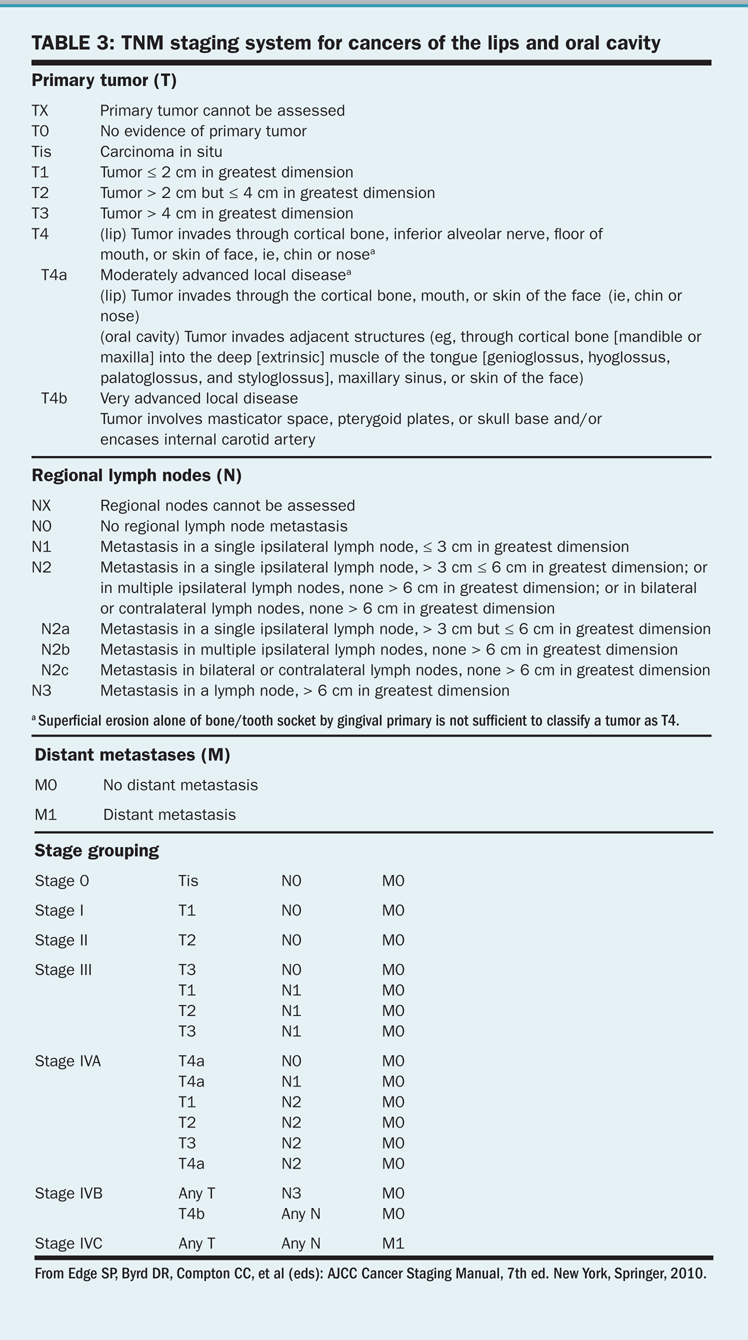
TNM staging system for cancers of the lips and oral cavity
Benign mucosal lesions include papillomas and keratoacanthomas, which may be exophytic or infiltrative. The exophytic lesions are less aggressive. The infiltrative papillomas and keratoacanthomas are more often associated with destruction of surrounding tissues and structures. These lesions may progress to malignancy. The TNM staging system for cancers of the lips and oral cavity is outlined in Table 3. T4 lesions have been divided into T4a (resectable) and T4b (unresectable) in the seventh edition of the AJCC Cancer Staging Manual.
Treatment
Management of cancers of the oral cavity involves surgery or radiotherapy for T1 or T2 lesions or combined-modality treatment that includes surgical resection and postoperative radiation therapy (60 to 70 Gy in 6 to 7 weeks) for advanced disease. For early-stage disease, surgery and radiotherapy are considered to have equivalent efficacy, although surgery is associated with less morbidity. Supraomohyoid neck dissection is performed in surgically treated patients with N0 necks. Bilateral neck dissection is performed if the tumor approaches the midline. Neck dissection is recommended for tumor thickness that is at least 4 mm, although some investigators believe that tumor thickness of 2 to 3 mm would be a more appropriate cutoff.
The lips
The lips are the most common site of oral cavity cancer. There are approximately 4,000 new cases of oral cavity cancer per year in the United States. The lower lip is affected most often. The vast majority of patients (90%) with lip cancer are men, and 33% have outdoor occupations.
Natural history. The most frequent presentation is a slow-growing tumor of the lower lip that may bleed and hurt. Physical examination must include assessment of hypoesthesia in the distribution of the mental nerve (cutaneous sensation of the chin area). Currently, less than 10% of American patients with squamous cell carcinoma of the lower lip have cervical metastases.
Treatment of primary tumor. Patients with early-stage lip cancers are usually treated with surgery. Radiation therapy may be used in patients who are medically unsuited for surgery or who refuse surgical resection.
Resection involves excision with at least 0.5 cm of normal tissue circumferentially beyond the recognized border of the tumor. After the resection of larger lesions, reconstruction may pose a major challenge. Small tumors are excised with a V incision.
Patients with advanced disease (stage III or IV) are usually managed with a combination of surgery and postoperative radiation therapy.
• The neck—Elective treatment of the neck is seldom recommended for patients with squamous cell carcinoma of the lower lip and a clinically negative neck, because few of these patients have cervical metastases. Neck dissection is recommended only in patients with palpable cervical metastases. Neck dissection is followed by postoperative radiation therapy.
• Results—The cure rate for T1–T3 tumors is 90% with surgical excision alone. Smaller lesions (T1–T2) may be treated equally well with radiation therapy. Survival rates for patients with T1 and T2 lesions are 90% and 80%, respectively. Overall, younger patients have a poorer prognosis, as do those with involvement of the mandible and extension of the tumor within the oral cavity.
The tongue
The oral tongue (anterior two-thirds) is the site of 75% of all tongue cancers. In 2012, approximately 12,770 men and women (9,040 men and 3,730 women) will be diagnosed with cancer of the tongue, and 2,050 will succumb to the disease.
Natural history. The most common presenting symptom in patients with cancer of the tongue is a persistent, nonhealing ulcer with or without associated pain. Other symptoms include difficulty with deglutition and speech. There may be a history of leukoplakia, especially in younger women.
• Rate of growth—Cancer of the tongue seems to grow more rapidly than other oral cavity cancers. Tongue cancers may grow in an infiltrative or exophytic fashion. The infiltrative tumors may be quite large at presentation.
• Lesion thickness—Thicker lesions have a worse prognosis than thin cancers, and lesion thickness is a more important prognostic factor than simple tumor stage. The incidence of clinically occult cervical metastases to the neck is significantly higher when the tumor thickness exceeds 4 mm. Nevertheless, tumor thickness has not been incorporated into the TNM staging system.
• Cervical metastases—Cervical metastases occur more frequently from tongue cancer than from any other tumor of the oral cavity. At initial evaluation, 40% of patients have node metastases.
Treatment of early-stage disease. Treatment usually entails partial glossectomy. Margins should be assessed at the time of resection, because the disease spreads along muscle bundles, leading to more extensive tumor than is appreciated grossly.
Radiation therapy using external-beam radiation or an interstitial implant is a suitable option for small or minimally infiltrating tumors. In general, morbidity appears to be less with surgery. Small cancers are resected without reconstruction. Following larger resections, reconstruction with a skin graft or with free tissue transfer produces good function.
Large, infiltrative lesions should be treated with combined-modality therapy (radiation therapy and surgical resection).
Treatment of advanced disease. More advanced tumors with mandibular involvement require composite resection, including a partial glossectomy, mouth floor resection, and mandibulectomy.
• The neck—A selective neck dissection is often recommended for clinically N0 neck cancer. Comprehensive neck dissection is required in the presence of palpable cervical metastases.
• Results—Control of disease closely correlates with the extent of the primary tumor and the presence of metastases. Rates of local tumor control using radiation therapy or surgery are similar for T1 (~85%) and T2 (~80%) tumors. T3 tumors should be treated using surgery and radiation therapy. Only 10% to 15% of local recurrences are amenable to repeated resection.
Overall survival is approximately 50%. Rates of survival at 5 years by stage are the following: stage I, 80%; stage II, 60%; and stages III and IV, 15% to 35%. For equivalent primary cancers, the presence of lymph node metastases decreases the survival rate by 50%.
The floor of the mouth
There are approximately 4,000 cases of floor of the mouth cancer in the United States annually. Mouth floor cancer accounts for 10% of head and neck cancer.
Pathology. Most lesions are moderately differentiated to well-differentiated squamous cell cancers and are exophytic.
Natural history. Patients usually present with a painful mass located near the oral tongue. Because these lesions do not cause pain until they are deep, they are frequently advanced at presentation.
Extension of disease into the soft tissues of the submandibular triangle is not uncommon. Fixation of the tumor to bone suggests possible mandibular involvement, which may be evaluated further with CT imaging. Changes in the mental foramen can be distinct or demonstrate slight asymmetry when compared with the contralateral anatomy. Restricted tongue mobility reflects invasion into the root of the tongue. Palpation demonstrates the depth of infiltration much better than does inspection alone.
Tumors near the midline may obstruct the duct of the submandibular gland, leading to swelling and induration in the neck, which may be difficult to distinguish from lymph node metastases. Level I nodes are the first-echelon metastatic sites.
• Multifocality—Multifocal cancers are more common in the floor of the mouth than in other oral cavity sites. Approximately 20% of patients with mouth floor tumors have second primary tumors, half of which are in the head and neck.
Treatment of early invasive lesions. Lesions (T1–T2) involving the mucosa alone may be treated with either surgery or irradiation alone with comparable results. Primary tumors with mandibular involvement should be surgically resected.
Cancer invades the mandible through tooth sockets. Hence, if the tumor merely abuts the mandible, a marginal mandibulectomy (which removes the bone margin but preserves continuity) may be performed. Otherwise, a segmental resection is needed. Selective neck dissection for treatment planning is advisable for thick stage I or II cancers.
Treatment of advanced disease. The treatment of choice for advanced disease is combined-modality therapy with surgery and radiation therapy. Complete surgical resection may require a composite resection of the mandible, including a partial glossectomy and neck dissection for advanced primary cancers. Lesions near the midline with a clinically positive lymph node require ipsilateral comprehensive neck dissection with a contralateral selective (supraomohyoid) neck dissection.
• Results—Overall, approximately 40% of patients are cured of their disease; 80% of recurrences appear within the first 2 years. Survival rates at 5 years by stage are the following: stage I, 85%; stage II, 75%; stage III, 66%; and stage IV, 30%. Signs of poor prognosis include involvement of both the tongue and mandible and extension of the tumor beyond the oral cavity.
Oropharynx
Carcinoma of the oropharynx affects approximately 8,000 patients in the United States annually. The incidence of oropharyngeal cancer is increasing. In the United States, over 60% of newly diagnosed oropharyngeal cancers are now attributable to HPV.
In ECOG 2399, a phase II trial, patients with locally advanced oropharyngeal and laryngeal cancers were treated with neoadjuvant paclitaxel and carboplatin followed by radiation with weekly paclitaxel. In 2008, Fakhry et al reported that patients who were HPV-positive had a higher response rate to both neoadjuvant chemotherapy and chemoradiation and had improved survival. For patients with HPV-positive tumors, HRs for progression and death were 0.27 and 0.36, respectively. These preliminary prospective data strongly confirmed previous retrospective analyses identifying HPV association as a favorable prognostic indicator, especially for oropharyngeal primary tumors.
Recursive partitioning analysis of RTOG 0129 revealed HPV status was the most important predictor of overall and progression-free survival. Patients with a history of smoking and high nodal burden had intermediate survival outcomes: not as favorable as those of HPV-positive non-smokers, but better than those of HPV-negative patients, according to a 2010 study by Ang et al. In addition, unlike smoking-related oropharyngeal cancer, HPV-related disease is not associated with a high incidence of second primary cancers. Future clinical trials will stratify oropharyngeal cancer patients based on this important risk factor.
Anatomy and pathology
The opening to the oropharynx is a ring bounded by the anterior tonsillar pillars (faucial arch), extending upward to blend with the uvula and inferiorly across the base of the tongue (behind the circumvallate papillae). The walls of the oropharynx are formed by the pharyngeal constrictor muscles, which overlie the cervical spine posteriorly. The superior boundary is the soft palate, which separates the oropharynx from the nasopharynx.
Subsites of the oropharynx include the base of the tongue, soft palate, tonsillar area, and posterior pharyngeal wall. The extent of a primary tumor may be difficult to assess because of its location.
The jugulodigastric nodes (levels II and III) constitute the first echelon of lymphatic drainage. Metastases may also appear in the parapharyngeal and retropharyngeal nodes and may be detected only through imaging studies.
Premalignant lesions occur in the oropharynx but are less common than in the oral cavity.
Treatment
Radiation therapy. External-beam radiation therapy (65 to 70 Gy over 7 weeks) and interstitial irradiation have been used in the curative treatment of oropharyngeal carcinomas for over 70 years. Radiation therapy represents a reasonable alternative to surgery and may also be required following radical resection of tumors with poor pathologic features to reduce the likelihood of local recurrence.
Altered fractionation schedules (accelerated and/or hyperfractionated) have gained interest in the past several decades based on both theoretical grounds and the results of mainly retrospective data. One prospective, randomized trial in patients with oropharyngeal cancer (excluding base of the tongue cancer) documented a 20% increase in locoregional tumor control and a 14% survival benefit at 5 years in patients who received a total dose of 8,160 cGy using 120 cGy bid 5 days per week in a hyperfractionated schedule, as opposed to 7,000 cGy in a conventionally fractionated schedule, specifically for intermediate-risk patients with T2 or T3 N0 or N1 squamous cell carcinomas. This improvement in outcome was not offset by any significant increase in acute or late tissue toxicity.
Local tumor control rates with radiation therapy alone for all primary sites (including the tonsils, soft palate, base of the tongue, and posterior oropharyngeal wall) are as follows: T1, 90%; T2, 80%; T3, 65%; and T4, 55%. Cancers of the tonsillar fossa are better controlled with irradiation than are cancers arising in other subsites of the oropharynx; this phenomenon may be related to tumors linked to HPV.
Chemoradiation therapy. A number of studies have been published supporting the use of multimodality therapy with radiation therapy and chemotherapy as an alternative to surgery with postoperative irradiation or irradiation alone. In an Intergroup trial by Adelstein et al, cisplatin (100 mg/m2) given every 3 weeks with standard irradiation was found to be safe and effective. Induction therapy followed by concurrent chemoradiation regimens has the potential for diminishing distant failure while maintaining the local tumor control achieved via concurrent regimens. Such regimens are currently under active investigation.
Surgery. Surgical extirpation of relatively inaccessible tumors in the oropharynx traditionally required radical surgery, including mandibulotomy or mandibulectomy, and occasionally laryngectomy. These surgical procedures were associated with worse functional outcomes relative to nonsurgical approaches. Radical surgery, however, has been supplanted by minimally invasive approaches. Using bivalved laryngoscopes for tumor exposure, surgical resection using the carbon dioxide (CO2) laser coupled to a microscope has led to complete pathologic resections and high local control rates for oropharyngeal cancer. The reported functional results have been good, with few patients requiring placement of a feeding tube or tracheotomies.
The use of the line-of-sight CO2 laser beam has been limited by inadequate exposure of the pathologic lesion in patients with short or stiff necks, retrognathia, full dentition, trismus, or obesity. Limited access to pathology has precluded use of transoral CO2 laser surgery in many patients. A flexible, photonic, band-gap fiber for the delivery of CO2 laser energy, developed by OmniGuide Inc, allows video-assisted visualization and extirpation of previously poorly accessible pathology. Limited access has also been surmounted by adaptation of the surgical robot for transoral applications. The daVinci Surgical System (Intuitive Surgical; Sunnyvale, California), originally designed as a three-armed robotic device controlling “wristed” surgical microinstruments and angled telescopes, has now been FDA-approved for a variety of general, cardiac, gynecologic, and urologic procedures. Improved surgical exposure to tumors in the upper aerodigestive tract has been reported relative to that obtained with more traditional transoral approaches. In 2009, transoral robotic surgery received FDA approval for T1 and T2 oropharyngeal lesions. Minimally invasive surgery is safe and effective for early-stage disease (stages I and II) and is under active investigation for advanced-stage disease. In 2011, Cohen et al published their experience using transoral robotic surgery in the treatment of 37 HPV-positive and 13 HPV-negative oropharyngeal cancer patients. Negative surgical resection margins were achieved in over 90% of patients. Extracapsular extension was identified in 44% of patients with HPV-positive cancers, and 25% of HPV-negative cancers. Two-year disease-specific and overall survival was 89.5% and 100%, and 81% and 80% for HPV-positive and HPV-negative patients, respectively. In this cohort, the most common cause of disease failure was distant metastases. One year after transoral resections, patient speech and swallowing scores approached pretreatment values.
The base of the tongue
Cancer of the base of the tongue is far less common than that of the oral tongue, but its incidence is increasing.
Anatomy. The base of the tongue is bordered anteriorly by the circumvallate papillae and posteriorly by the epiglottis. There is a rich lymphatic network, with metastases frequently seen in levels II to V.
Natural history. The base of the tongue is notorious for lesions that infiltrate deeply into muscle and are advanced at diagnosis. This finding is probably due to the relatively asymptomatic anatomic location. Thus, bimanual oral examination with digital palpation is a critical part of the physical examination.
Most patients present with pain and dysphagia. Other symptoms include a neck mass, weight loss, otalgia, and trismus.
All oropharyngeal cancers have a strong propensity to spread to the lymph nodes, and tumors arising at the base of the tongue are no exception. Approximately 70% of patients with T1 primary base of the tongue tumors have clinically palpable disease in the neck, and 20% to 30% have palpable, bilateral lymph node metastases. The risk of nodal metastases increases with increasing T stage and approaches 85% for T4 lesions.
Treatment of early-stage disease. Stage I or II cancers may be treated equally effectively with either surgical resection or radiation therapy alone. If irradiation of the primary tumor is employed, both sides of the neck should be treated, even if the nodes do not seem to be involved. Surgical management of an oropharyngeal malignancy includes lymphadenectomy. For patients clinically staged N0, selective neck dissection encompassing levels II–IV is sufficient. For patients with cancers at the base of the tongue, bilateral neck dissection is required.
Treatment of advanced disease. More advanced disease may require total resection of the tongue base with or without laryngectomy to ensure complete removal of disease. Total resection of the tongue base is associated with severe oropharyngeal dysphagia with aspiration, but even subtotal resection of the base of the tongue may result in significant aspiration, which is exacerbated by postoperative radiotherapy. Total laryngectomy may be the only way to isolate the airway from oral secretions and eliminate the risk of aspiration. Chemoradiation therapy via external-beam irradiation or irradiation alone combined with an implant can be curative for patients with advanced tumors of the tongue base.
• Results—In general, the prognosis of cancers of the tongue base is poor because of their advanced stage at presentation. The extent of nodal disease predicts survival. For T1 and T2 cancers, local tumor control rates approach 85%. The major determinant of treatment failure is the tumor’s growth pattern, with a high local tumor control rate for exophytic lesions and a far worse rate for infiltrative tumors.
Tonsils and tonsillar pillar
The tonsils and tonsillar pillar are the most common locations for tumors of the oropharynx.
Natural history. Tonsillar fossa tumors tend to be more advanced and more frequently metastasize to the neck than do tonsillar pillar cancers. At presentation, 55% of patients with fossa tumors have N2 or N3 disease, and contralateral metastases are common. Symptoms include pain, dysphagia, weight loss, a mass in the neck, and trismus.
Treatment. Single-modality therapy (irradiation or surgery alone) is acceptable for T1 and T2 tumors. Irradiation alone may be curative for more advanced tumors, although chemotherapy is often added in a concurrent fashion for T3 or T4 disease or in the setting of N2 or N3 disease. The neck should always be included in treatment planning. More advanced disease usually requires surgery combined with irradiation. Well-lateralized cancers of the tonsillar fossa (minimal to no involvement of the soft palate or base of the tongue) may be treated with unilateral irradiation, since contralateral neck failure is rare (< 10%).
Hypopharynx
Hypopharyngeal cancers are approximately one-third as common as laryngeal cancers.
Anatomy
The hypopharynx (or laryngopharynx) is the entrance to the esophagus. The superior aspect (above the plane of the hyoid bone) communicates with the oropharynx, and the inferior border is situated in the plane of the lowest part of the cricoid cartilage (the esophageal inlet). The anterior surface (postcricoid area) is contiguous with the posterior surface of the larynx, adjacent to the lamina of the cricoid cartilage. The pharyngeal musculature forms the lateral and posterior walls. The piriform sinuses are within the hypopharynx on each side of the larynx.
The hypopharynx contains three subsites: the paired piriform sinuses (lateral, pear-shaped funnels); the posterior pharyngeal wall, from the level of the vallecula to the level of the cricoarytenoid joints; and the postcricoid area (pharyngoesophageal junction), which begins just below the arytenoids and extends to the inferior border of the cricoid cartilage. The piriform sinuses are composed of a medial wall, which abuts the aryepiglottic fold, and a lateral wall. Seventy percent of hypopharyngeal cancers occur in the piriform sinuses.
Natural history
Hypopharyngeal tumors produce few symptoms until they are advanced (> 70% are stage III or IV at presentation). They may cause a sore throat, otalgia, a change in voice, odynophagia, or an isolated neck mass. Subtle changes on physical examination, including pooling of secretions, should be regarded with concern.
Nodal metastases. Diffuse local spread is common and is due to tumor extension within the submucosa. Abundant lymphatic drainage results in a higher incidence of lymph node metastases than with other head and neck tumors. At presentation, 70% to 80% of patients with hypopharyngeal tumors have palpable cervical lymph node metastases; in half of these patients, palpable cervical nodes are the presenting complaint. Levels II and III are most commonly involved. Bilateral metastases are seen in only 10% of patients with piriform sinus cancers but in 60% of those with postcricoid tumors.
Synchronous lesions. These are common. Overall, 20% to 25% of patients with hypopharyngeal cancer develop a second primary tumor within 5 years, usually in the head and neck.
Chemotherapy and external-beam radiotherapy. Similar in design to the VA Cooperative Laryngeal Cancer Study, a randomized EORTC trial has shown that initial therapy with cisplatin and 5-FU, followed by definitive irradiation in patients with complete remissions (or, alternatively, surgical salvage), results in at least equivalent survival relative to immediate pharyngolaryngectomy. Of patients treated with initial chemotherapy, 28% retained a functional larynx at 3 years (approximately two-thirds of survivors).
Though directed to laryngeal cancers (rather than hypopharyngeal cancers), the RTOG 91-11 trial results have been widely interpreted as applying to hypopharyngeal cancer, increasing enthusiasm for concomitant chemoradiation therapy for patients with advanced cancers of the hypopharynx.
Transoral laser surgery for piriform sinus carcinoma. Organ-sparing approaches such as transoral laser microsurgery for piriform sinus carcinomas have been used in several institutions for the past 25 years. Long-term follow-up in a retrospective review of 129 previously untreated patients has been published. In this series, which included mostly patients with advanced-stage disease, the reported 5-year local control rate was 82% for patients with stages I and II cancers and 69% for those with stages III and IV cancers. Radiation therapy was administered after surgery. Laryngeal preservation rates were comparable to the local tumor control rates. The 2-year overall survival rates were 91% for patients with stages I and II cancer and 75% for patients with stages III and IV cancer. These oncologic and functional results compare favorably with those obtained with either nonsurgical or surgical approaches requiring opening the neck and pharynx.
Larynx
Laryngeal cancers constitute approximately 1.2% of all new cancer diagnoses in the United States. Approximately 12,360 new cases are expected in 2012, and it is estimated that 3,650 men and women will die of the disease.
Anatomy and pathology
The laryngeal anatomy is complex and includes cartilages, membranes, and muscles. The three subsites of the larynx are the glottis, or true vocal cords; the supraglottis, which includes the false cords, epiglottis, and aryepiglottic folds; and the subglottis, which is the region below the glottis and within the cricoid cartilage. The TNM staging system for cancers of the larynx is outlined in Table 4.
Treatment
Surgery. Surgical treatment for laryngeal cancer includes transoral or open approaches. Treatment is dictated by the site and extent of the lesion. All or part of the larynx may need to be removed to achieve surgical control of laryngeal cancer. Decision making for partial laryngectomy is complex and depends on the patient’s overall health, the extent of local disease, the skill of the surgeon, and patient preference.
External-beam radiation therapy and chemoradiation therapy. With improvements in techniques and fractionation schedules, external-beam radiation therapy, which allows for laryngeal preservation, is an option for all but the most advanced tumors.
• Results—As in other head and neck sites, patients with early laryngeal cancer may be treated with surgery or radiation therapy, and those with advanced-stage disease require multimodal therapy, including surgery and postoperative radiation therapy or chemotherapy and radiation therapy. For T1 and T2 tumors of the glottic or supraglottic larynx, radiation therapy is associated with local tumor control rates of 75% to 95%. Open partial laryngectomy for early laryngeal cancer is associated with local tumor control rates of 84% to 98% but requires a temporary tracheotomy and may be associated with postoperative vocal and swallowing dysfunction. Transoral laser surgery for early laryngeal cancer has yielded local tumor control rates of 82% to 100%, without significant dysphagia or a need for a temporary tracheotomy. However, the technical expertise required to perform transoral laser surgery is limited to a small number of institutions.
Select patients with T3 or T4 laryngeal cancers may be candidates for larynx preservation surgery, but most require total laryngectomy as the surgical option. The VA Cooperative Laryngeal Cancer Study was the first trial to test the efficacy of chemotherapy and radiation therapy in the management of stages III and IV laryngeal cancer, assess the possibility for laryngeal preservation using such a regimen, and compare its efficacy against the historic standard of surgery and postoperative radiation therapy. The VA Cooperative Laryngeal Cancer Study randomized patients with resectable squamous cell carcinoma of the larynx to receive either total laryngectomy followed by radiation therapy or neoadjuvant therapy with cisplatin and 5-FU followed by radiation therapy for those achieving a good response to chemotherapy.
Approximately two-thirds of patients survived 2 years following the combination of either chemotherapy plus irradiation or resection plus irradiation. Of patients initially treated with chemotherapy and irradiation, one-third required total laryngectomy because of a lack of response to treatment and in the remaining two-thirds the larynx was successfully preserved. In this study, the increased local recurrence rate in the chemoradiation therapy group was offset by the decreased incidence of distant metastases and second primary tumors, and the 2-year survival rate was comparable between the groups. Advanced T stage was a risk factor for local failure in this study. Salvage total laryngectomy was required for 56% of T4 laryngeal cancers, suggesting that patients with bulky primary tumors may not be optimal candidates for organ-preservation therapy.
Data from the RTOG 91-11 trial demonstrated an improvement in locoregional tumor control using concurrent chemoradiation therapy relative to induction chemotherapy followed by radiotherapy or radiotherapy alone. There was no primarily surgical or altered fractionated arm in the study. This three-armed study randomized 517 patients with stages III and IV laryngeal cancer to receive one of the following regimens: induction chemotherapy (with cisplatin/5-FU) followed by radiation therapy or concurrent chemoradiotherapy (with cisplatin given on days 1, 22, and 43) or radiation therapy alone. Locoregional tumor control for the three arms was 61%, 78%, and 56%, respectively. The larynx preservation rate was 75%, 88%, and 70%, respectively. At 2 years, the proportion of patients in whom the larynx was preserved after irradiation and concurrent cisplatin (88%) differed significantly from the proportion in groups given induction chemotherapy followed by irradiation (75%; P = .005) or irradiation alone (70%; P < .001). The authors concluded that the combination of irradiation and concurrent cisplatin was superior to induction chemotherapy followed by irradiation or irradiation alone for laryngeal preservation and locoregional tumor control. Notably, patients with T4 laryngeal cancers accounted for only 10% of patients in this study, compared with 25% in the original VA Cooperative Laryngeal Cancer Study, accounting for the improved local tumor control with surgery. Follow-up studies revealed that local failure nevertheless remained the predominant initial site of failure, and the survival of patients who underwent salvage laryngectomy was somewhat diminished.
The cure rate for early cancers of the larynx approaches 80% or more. Half of patients with T3 cancer are cured, whereas more than two-thirds of patients with T4 cancer will die of the disease.
Supraglottis
Supraglottic tumors occur less frequently than tumors of the true vocal cords. The epiglottis is the most common location for supraglottic cancers.
Natural history
Tumors close to the glottis produce symptoms earlier than do tumors at other subsites, usually hoarseness. In contrast, nearly 60% of patients with supraglottic tumors have T3 or T4 primary tumors at presentation.
The supraglottis has a rich lymphatic network. There is an associated high incidence of lymph node metastases in early-stage tumors (40% for T1 tumors). The incidence of metastases in patients with clinically N0 neck cancer is about 15%. The incidence of bilateral cervical lymph node involvement is about 10%, and this rate increases to 60% for anterior tumors. The neck is a frequent site of recurrence in patients with supraglottic malignancies.
Treatment. Appropriate cancers may be treated with partial laryngectomy. Supraglottic laryngectomy removes the upper portion of the thyroid cartilage and its contents, including the false vocal cords, as well as the epiglottis and aryepiglottic folds. This approach preserves speech and swallowing, but more extensive resections are not well tolerated by patients with impaired lung function who are not able to tolerate the inevitable postoperative aspiration. Supracricoid partial laryngectomy is suitable for supraglottic tumors that cross the ventricle to involve the glottis. Select T3 tumors with limited pre-epiglottic space involvement may be approached with this procedure. Patients must have sufficient pulmonary reserve to be able to tolerate the chronic aspiration associated with this procedure. The local tumor control rates of transoral laser supraglottic laryngectomy are between 75% and 100% for T1 and T2 lesions in recent series and are associated with less postoperative dysphagia than open approaches. Significant technical expertise is needed, and patients must have the proper habitus to ensure adequate exposure.
The high incidence of cervical metastases makes treatment of the neck a necessity. About one-third of cases of clinically negative neck cancer contain involved nodes, and the incidence of recurrence in untreated patients is high. For patients undergoing surgical treatment of T1 or T2 primary tumors, bilateral selective neck dissection is advisable.
Supraglottic laryngectomy is seldom appropriate as salvage therapy following irradiation because of complications, including swelling, difficulty in swallowing, and poor wound healing. The usual salvage operation for persistent supraglottic cancer following radiation therapy is total laryngectomy. However, some patients remain candidates for larynx-preservation surgery.
Advanced-stage laryngeal cancer usually requires multinodal treatment, as noted previously. Laryngeal preservation frequently is not possible using primary surgical approaches. The use of primary radiation enables preservation of the larynx. The use of combined radiation concurrently with chemotherapy is the treatment of choice for many patients.
Glottis
The glottis is the most common location of laryngeal cancer in the United States, accounting for more than half of all cases. The incidence of laryngeal cancers and other malignancies related to smoking has been declining.
Natural history
The cure rate for tumors of the true vocal cords is high. These cancers produce symptoms early and, thus, most are small when detected. Approximately 60% are T1 and 20% are T2. Normal cord mobility implies invasion of disease limited to the submucosa. Deeper tumor invasion results in impaired vocal cord motion; this finding is most common in the anterior two-thirds of the vocal cord.
The true vocal cords have very little lymphatic drainage. Cervical metastases are infrequent with T1 (1%) and T2 tumors (3% to 7%).
Treatment
Carcinoma in situ is highly curable and may be treated equally well with microexcision, laser vaporization, or radiation therapy. Treatment decisions should be based on the extent of local disease. Serial recurrences should heighten suspicion of an invasive component, and a more aggressive approach, such as partial or total laryngectomy or irradiation, should be employed. Hypofractionated (> 2 Gy/d) radiotherapy is preferred to conventional fractionation (1.8 to 2 Gy). Local tumor control for T1 using surgery or irradiation is comparable, usually greater than 90%. Local tumor control for T2 glottic lesions is reported to be between 70% and 85% with radiotherapy and 85% and 95% with open partial laryngectomy. Partial laryngectomy can be performed in select patients after irradiation failure of some T1 or T2 glottic cancers.
Advanced T4 disease is best treated with total laryngectomy. Most T3 lesions are now being treated with concomitant chemoradiotherapy, with salvage laryngectomy required in approximately 20% of patients for residual/recurrent disease or laryngeal dysfunction.
Results. Cure rates by tumor size alone are as follows: T1, 90%; T2, 80%; T3, 50%; and T4, 40%. Neck involvement worsens the prognosis dramatically.
Subglottis
Subglottic cancer is unusual, accounting for less than 10% of all laryngeal cancers.
Natural history
These cancers tend to be poorly differentiated and, because the region is clinically “silent,” most present as advanced lesions (~70% are T3–T4). The subglottis also has rich lymphatic drainage, and the incidence of cervical metastases is 20% to 30%.
Treatment
Partial laryngectomy is not practical for the treatment of tumors in the subglottis and, thus, therapy usually includes total laryngectomy plus neck dissection. Combination therapy (surgery plus radiation therapy [60 to 65 Gy in 6 to 7 weeks]) is recommended for patients with more advanced disease.
Results. The cure rate for the uncommon T1 and T2 tumors is approximately 70%. Most failures occur in the neck. The cure rate for more advanced lesions is approximately 40%.
Unknown Head and Neck Primary Site
The cervical lymph nodes are the most common metastatic site at which squamous cell carcinoma is found.
Natural history
Most patients who present with squamous cell carcinoma involving cervical lymph nodes, especially in the upper or middle portion of the cervical chain, will have a primary site within the head and neck. When the lower cervical or supraclavicular lymph nodes are involved, a primary lung cancer should be suspected.
In the overwhelming majority of these cases, the primary lesion will be discovered on the basis of the history; physical examination; proper radiographic evaluation (CT and/or MRI); and examination with the patient under anesthesia, with endoscopy (direct laryngoscopy and nasopharyngoscopy), targeted biopsies, and tonsillectomy. Esophagoscopy and bronchoscopy seldom yield a diagnosis in patients with upper cervical lymph node involvement. “Silent” primary tumors are most often discovered in the base of the tongue or within tonsillar crypts. Tonsillectomy and/or base of tongue mucosectomy may identify the primary site, obviating the need for wide-field mucosal irradiation which otherwise includes the nasopharynx, oropharynx, and hypopharynx. Lymph node metastases should be tested for the presence of HPV/p16 and EBV.
Treatment
A substantial percentage of patients achieve long-term disease-free survival after treatment of the involved side of the neck. Locoregional control and survival are diminished by multiple lymph nodes and the presence of extracapsular extension of disease in the involved neck.
Irradiation alone. Patients with early-stage neck disease (N1 disease) can be treated with surgery alone if an open biopsy has not been performed. Recurrences in mucosal sites occur in 20% to 30% of cases, and radiotherapy is used to lower the risk of recurrence in the neck, not the primary. Radiation therapy dosages and techniques should be similar to those used in patients with early-stage (ie, T1) primary head and neck cancer. Dosages ranging from 5,760 to 6,480 cGy are acceptable. The nasopharynx and oropharynx, with or without the hypopharynx, should be included in the irradiated field.
Surgery alone. When neck dissection is used at the initial treatment, a primary tumor in the head and neck subsequently becomes obvious in about 20% of patients when radiation therapy is not employed.
Irradiation alone or combined with surgery. Combination therapy (surgery plus radiation therapy [60 to 65 Gy in 6 to 7 weeks]) is recommended for patients found at surgery to have multiple involved nodes or extracapsular extension or for those who have suspected residual microscopic disease in the neck without a clinically detectable tumor. Open nodal biopsy does not appear to compromise outcome as long as adequate radiotherapy is delivered subsequently.
In most modern series utilizing predominantly combination therapy, 5-year survival rates exceed 50%. The volume of tumor in the involved neck influences outcome, with N1 and N2 disease having a significantly higher cure rate than N3 disease or massive neck involvement. Regional relapse is usually predicted by extranodal disease.
Chemotherapy. The role of chemotherapy in treating patients with an unknown primary metastatic squamous cell carcinoma in cervical lymph nodes remains undefined. The benefit of concurrent chemoradiation therapy for these patients is uncertain (because their primary sites are small or absent), and neck control after resection followed by irradiation, or after irradiation alone, is excellent in patients with cancers of an unknown primary site.
Nasopharynx
Nasopharyngeal carcinoma is uncommon in most of the world. Endemic areas include southern China, northern Africa, and regions of the far Northern Hemisphere. The incidence (per 1,000 population) ranges from 25.6 in men and 10.2 in women in Hong Kong to 0.6 in men and 0.1 in women in Connecticut.
Epidemiology and risk factors
Gender and age. The incidence of nasopharyngeal cancer peaks in the fourth to fifth decades of life, and the male-female ratio is 2.2:1. Both patient age at disease onset and male-female ratio are lower for nasopharyngeal cancer than for other head and neck malignancies.
Risk factors. Nasopharyngeal carcinoma World Health Organization (WHO) types 2 and 3 (see section on “Anatomy and pathology”) appears to have different determinants than do other head and neck cancers. They include diet, viral agents, and genetic susceptibility. Populations of endemic areas have a diet characterized by high consumption of salt-cured fish and meat. Studies reveal an association between EBV and nasopharyngeal carcinoma. Anti-EBV antibodies have been found in the sera and saliva of patients with this type of carcinoma. Major histocompatibility (MHC) profiles associated with an increased relative risk include H2, BW46, and B17 locus antigens.
Anatomy and pathology
The nasopharynx communicates anteriorly with the nasal cavity and inferiorly with the oropharynx. The superior border is the base of the skull. The lateral and posterior pharyngeal walls are composed of muscular constrictors. Posteriorly, the nasopharynx overlies the first and second cervical vertebrae. The eustachian tubes open into the lateral walls. The soft palate divides the nasopharynx from the oropharynx.
Cancers arising in the nasopharynx are classified using WHO criteria: type 1 denotes differentiated squamous cell carcinoma; type 2, nonkeratinizing carcinoma; and type 3, undifferentiated carcinoma. The TNM staging system for cancers of the pharynx is outlined in Table 5.
Natural history
A mass in the neck is the presenting complaint in 90% of patients. Other presenting symptoms include a change in hearing, sensation of ear stuffiness, tinnitus, nasal obstruction, and pain.
Cranial nerve involvement. Invasion of disease into the base of the skull is seen in approximately 25% of cases and may lead to cranial nerve involvement. CN VI is the first cranial nerve to be affected, followed by CN III and CN IV. Deficits are manifested by changes in ocular motion. Involvement of CN V may also occur; this is manifested by pain or paresthesia high in the neck or face.
Level V metastases. Unlike malignancies of the oral cavity and oropharynx, nasopharyngeal cancers often metastasize to level V lymph nodes. Bilateral metastases are common.
Treatment
Treatment of nasopharyngeal cancer usually involves radiation therapy for the primary tumor and draining lymph nodes. Overall survival is 50% at 5 years. Surgical resection has high morbidity and is seldom entertained.
Nasopharyngeal cancer is distinguished from other sites of head and neck cancer by its radiosensitivity and chemosensitivity. Advanced nodal disease can be controlled by irradiation alone in approximately 50% of patients, but eventual distant metastasis remains a problem.
The final report of the Intergroup trial 0099 confirmed that for patients with locally advanced nasopharyngeal cancer, concurrent cisplatin chemotherapy with radiation therapy (followed by systemic chemotherapy) provided a clear survival benefit when compared with treatment with irradiation alone. At 5 years, patients who received combined-modality therapy had an overall survival rate of 67%, compared with 37% with radiation therapy alone (P = .001). Disease-free survival at 5 years was 74% for the chemoradiation therapy arm vs 46% for the arm that received radiation therapy alone. Treatment of metastatic disease involves cytotoxic therapy, often with a platinum doublet. For tumors associated with EBV, approaches with immunotherapy are an active area of research.
Recurrent Head and Neck Cancer
As mentioned previously, surveillance after treatment of head and neck cancer is mandatory, because early detection of second primary cancers or locoregional recurrence affords the best chance for disease control. Nearly two-thirds of patients whose head and neck cancer recurs develop a tumor at (or near) the primary site or in the neck nodes. Eighty percent of head and neck cancer recurrences eventuate within 2 years.
Differentiating Recurrence From Late Complications of Irradiation
Differentiating between recurrent carcinoma and significant sequelae of radiotherapy is a difficult clinical problem at all sites within the head and neck. Any suspicious mucosal changes, enlarged nodes in the neck, or discrete subcutaneous nodules warrant prompt biopsy.
Candidates for Surgery
Different choices of first treatment (ie, surgery or radiation therapy) and the intensity of follow-up influence success in treating recurrence. Aggressive surgical intervention should be offered to two groups of patients with recurrent local or regional disease: those whose therapy is chosen with curative intent and those with the prospect of significant palliation.
The types of recurrence that may be approached surgically with the greatest likelihood of success include (1) metastases in the neck after initial treatment limited to the primary tumor alone and (2) reappearance or persistence of cancer at a site previously treated with radiotherapy alone. Salvage resection may also be considered in other situations, however, including, for example, the appearance of cancer in the neck after prior irradiation or neck dissection, at the margins after previous resection, and even at the base of the skull.
After surgery for head and neck cancer, patients remain at high risk for locoregional recurrence. In one study, 130 patients who had undergone surgery for recurrent disease were randomized to observation or to postoperative reirradiation combined with concomitant hydroxyurea and 5-FU. A higher incidence of treatment-related mortality and severe acute and chronic toxicity was found in the treatment group. The disease-free, but not overall, survival was improved in the treatment arm (P = .006 and .5, respectively).
Surgery is the standard of care for the treatment of recurrent disease, but there is a growing body of evidence suggesting that reirradiation with concurrent chemotherapy can cure select patients when resection is not possible. Several institutions have reported experiences re-treating patients, and these results led to the development of the first multi-institution reirradiation study.
A single-arm, phase II study (RTOG 96-10) evaluated toxicity and therapeutic results for patients with recurrent squamous cell carcinoma of the head and neck. Eighty-six patients received four weekly courses of 1.5-Gy fractions twice daily with concurrent 5-FU and hydroxyurea. Each cycle was separated by 1 week of rest. The median survival was 8.1 months, and the 1- and 2-year survival rates were 41.7% and 16.2%, respectively. Compared with patients who experienced early recurrences, patients whose disease recurred 3 years after the original irradiation fared better, with 1- and 2-year survival rates of 48.1% and 32.1%, respectively.
The first results for the entire cohort of patients for RTOG 99-11, the successor trial to RTOG 96-10, were presented in 2005. In this study, patients with locally recurrent or second primary head and neck tumors who previously received radiation therapy were treated with split-course hyperfractionated radiotherapy (60 Gy total; 1.5 Gy/fraction twice daily for 5 days every 2 weeks for four cycles) in combination with cisplatin (15 mg/m2 IV daily) for five courses and paclitaxel (20 mg/m2 IV daily) for five courses every 2 weeks for four cycles. Granulocyte colony-stimulating factor (G-CSF) support was administered on days 6 through 13 of each 2-week cycle. Of the 105 patients enrolled, 99 were eligible for analysis, and 23% of the patients had second primary head and neck tumors. The median prior dose of radiotherapy was 65.4 Gy (range, 45 to 75 Gy), and the median time from prior radiotherapy was 40 months.
Of eight patients with grade 5 (fatal) toxicities, five occurred during the acute period (dehydration, pneumonitis, neutropenia [2 cases], and cerebrovascular accident) and three during the late period (two of three attributable to carotid hemorrhage). Other acute toxicities included leukopenia (30% grade 3/4), anemia (21% grade 3/4), and GI toxicity (48% grade 3/4). The median follow-up for patients was 23.6 months, and the median survival was 12.1 months.
The estimated 1- and 2-year overall survival rates were 50.2% and 25.9%, respectively. Median survival time and the 1-year progression-free survival rate were 7.8 months and 35%, respectively. Overall survival was significantly better (P = .044) than for the historic control in RTOG 96-10 (estimated 1- and 2-year overall survival rates, 41.7% and 16.7%, respectively).
Despite significant toxicity and high mortality, hyperfractionated split-course reirradiation with concurrent cisplatin and paclitaxel chemotherapy proved feasible in this select patient population. This approach was to be tested in RTOG 04-21, a phase III trial randomizing patients between reirradiation with concurrent cisplatin and paclitaxel chemotherapy vs chemotherapy alone; however, this trial was closed in early 2007 because of a lack of accrual.
Unless a patient cannot tolerate an operation, resection of discrete local or regional recurrent tumors should be entertained as the first course of treatment. Management of recurrences involves complex decision making and requires familiarity with multidisciplinary care.
Suggested Reading
Ang KK, Harris J, Wheeler R, et al: Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24-35. 2010.
Ang K, Pajak T, Wheeler R, et al: A phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas (RTOG 1209): Report of efficacy and toxicity. 51st Annual ASTRO Meeting; November 1–5, 2009; Chicago, Illinois. Abstract LB2.
Bernier J, Cooper JS Pajak TF, et al: Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (#9501). Head Neck 27:843–850, 2005.
Bonner JA, Harari PM, Giralt J, et al: Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567–578, 2006.
Cohen EEW, Karrison T, Kocherginsky M, et al: DeCIDE: A phase III randomized trial of docetaxel (D), cisplatin (P), 5-fluorouracil (F) (TPF) induction chemotherapy (IC) in patients with N2/N3 locally advanced squamous cell carcinoma of the head and neck (SCCHN). 2012 ASCO Annual Meeting; June 1-5, 2012; Chicago, Illinois. Abstract 5500.
Cohen MA, Weinstein GS, O’Malley BW, et al: Transoral robotic surgery and human papillomavirus status: Oncologic results. Head Neck 33:573–580, 2011.
Cooper JS, Pajak TF, Forastiere AA, et al: Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 350:1937–1944, 2004.
Eisbruch A, Harris J, Garden AS, et al: Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00-22). Int J Radiat Oncol Biol Phys 76:1333–1338, 2010.
Fakhry C, Westra WH, Li S, et al: Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100:261–269, 2008.
Forastiere AA, Goepfert H, Maor M, et al: Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 349:2091–2098, 2003.
Garden AS, Morrison WH, Rosenthal DI, et al: Patterns of disease recurrence following radiation for oropharyngeal cancer. Int J Radiat Oncol Biol Phys 75:S32, 2009.
Haddad RI, Rabinowits G, Tishler RB, et al: The PARADIGM trial: A phase III study comparing sequential therapy (ST) to concurrent chemoradiotherapy (CRT) in locally advanced head and neck cancer (LANHC). 2012 ASCO Annual Meeting; June 1-5, 2012; Chicago, Illinois. Abstract 5501.
Handra-Luca A, Hernandez J, Mountzios G, et al: Excision repair cross complementation group 1 immunohistochemical expression predicts objective response and cancer-specific survival in patients treated by cisplatin-based induction chemotherapy for locally advanced head and neck squamous cell carcinoma. Clin Cancer Res 13:3855-3859, 2007.
Horwitz EM, Harris J, Langer CJ, et al: Combination with split course concomitant hyperfractionated re-irradiation in patients with recurrent squamous cell cancer of the head and neck: Results of RTOG 99-11. Int J Radiat Oncol Biol Phys 63(suppl 1):S72–S73, 2005.
Janot F, de Raucourt D, Benhamou E, et al: Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. J Clin Oncol 26:5518-5523, 2008.
Jun H, Ahn M, Kim H, et al: Clinical significance of ERCC1 expression in advanced squamous cell carcinoma of the head and neck treated with cisplatin-based concurrent chemoradiation. J Clin Oncol 25(18S):6061, 2007.
Kies MS, Harris J, Rotman MZ, et al: Phase II randomized trial ofpostoperative chemoradiation plus cetuximab for high-risk squamous cell carcinoma of the head and neck (RTOG 0234). Int J Radiat Oncol Biol Phys 75:S14–S15, 2009.
Khuri FR, Lee JJ, Lippman SM, et al: Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst 98:441-450, 2006.
Leonhardt FD, Quon H, Abraho M, et al: Transoral robotic surgery for oropharyngeal carcinoma and its impact on patient-reported quality of life and function. Head Neck Apr 5, 2011 [Epub ahead of print].
Lonneux M, Hamoir M, Maingon P, et al: Positron emission tomography with [18F] fluorodeoxyglucose improves staging and patient management in patients with head and neck squamous cell carcinoma: A multicenter prospective study. J Clin Oncol 28:1190–1195, 2010.
Moeller BJ, Rana V, Cannon BA, et al: Prospective risk-adjusted [18F]Fluorodeoxyglucose positron emission tomography and computed tomography assessment of radiation response in head and neck cancer. J Clin Oncol 27:2509-2517, 2009.
O’Malley BW Jr, Weinstein GS, Snyder W, et al: Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope 116:1465–1472, 2006.
Pignon JP, le Maitre A, Bourhis J: Meta-analyses of chemotherapy in head and neck cancer (MACH-NC): An update. Int J Radiat Oncol Biol Phys 69:S112–S114, 2007.
Posner MR, Hershock DM, Blajman CR, et al: Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 357:1705–1715, 2007.
Siegel R, Naishadham D, Jemal A: Cancer statistics, 2012. CA Cancer J Clin 62:10–29, 2012.
Steiner W, Ambrosch P, Hess CF, Kron M. Organ preservation by transoral laser microsurgery in piriform sinus carcinoma. Otolaryngol Head Neck Surg 124:58-67, 2001.
Trotti A, Fu KK, Pajak TF, et al: Long-term outcomes of RTOG 90-03: A comparison of hyperfractionation and two variants of acclerated fractionation to standard fractionation radiotherapy for head and neck squamous carcinoma. Int J Radiat Oncol Biol Phys 63:S70–S71, 2005.
Urba S, Wolf G, Eisbruch A, et al: Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: A new treatment paradigm. J Clin Oncol 24:593–598, 2006.
Vermorken J, Mesia R, Vega V, et al: Cetuximab extends survival of patients with recurrent or metastatic SCCHN when added to first line platinum based therapy: Results of a randomized phase III (Extreme) study. J Clin Oncol 25(18S):6091, 2007.
Weinberger PM, Yu Z, Kountourakis P, et al: Defining molecular phenotypes of human papillomavirus-associated oropharyngeal squamous cell carcinoma: Validation of three-class hypothesis. Otolaryngol Head Neck Surg 141:382-389, 2009.
Yom SA, Machtay M, Biel MA, et al: Survival impact of planned restaging and early surgical salvage following definitive chemoradiation for locally advanced squamous cell carcinomas of the oropharynx and hypopharynx. Am J Clin Oncol 28:385–392, 2005.
Source: www.cancernetwork.com