Liver, Gallbladder, and Biliary Tract Cancers
By Lawrence D. Wagman, MD1, John M. Robertson, MD2, Laura Raftery, MD3, Bert O’Neil, MD3, Keeran R. Sampat, MD4 | 8 Μάρτιος 2013
1Division of Surgery, The Center for Cancer Prevention and Treatment, St. Joseph Hospital 2Department of Radiation Oncology, William Beaumont Hospital 3Division of Hematology/Oncology, University of North Carolina at Chapel Hill 4Division of Hematology/Oncology, UNC Lineberger Comprehensive Cancer Center
HEPATOCELLULAR CANCER
Worldwide, hepatocellular carcinoma is the fifth most common malignancy and the third most common cause of cancer mortality. Most patients with hepatocellular carcinoma suffer from cirrhosis, primarily caused by alcoholism or chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV); decades may pass between infection with viral hepatitis and development of this cancer. The approximately equal annual incidence vis-à-vis mortality of 1 million persons reported around the world stands as evidence of its lethality.
Epidemiology
Gender
Hepatocellular carcinoma is the most common tumor in males worldwide, with a male to female ratio of 5:1 in Asia and 2:1 in the United States.
Geography
Eighty percent of new hepatocellular cancer cases occur in developing countries, but the incidence of this cancer is also rising in developed countries. Modeling of the spread of HCV infection suggests that this number may continue to increase dramatically. In the United States, the incidence of hepatocellular carcinoma is 5 per 100,000, whereas in East Asia and sub-Saharan Africa, this neoplasm occurs at an incidence of 150 per 100,000 population and accounts for almost 50% of all diagnosed tumors.
Age
The incidence of hepatocellular cancer increases with age. The mean age at diagnosis is 53 years in Asia and 67 years in the United States.
Race
From 2003 to 2005, the incidence of hepatocellular tumors in the United States was higher among Asian immigrants (12 per 100,000) and black individuals (7 per 100,000) than among white individuals (4 per 100,000). However, a study analyzing the Surveillance, Epidemiology, and End Results (SEER) data has shown that the incidence of hepatocellular carcinoma is rising in both white and black populations in the United States.
Survival
In patients who meet stringent criteria and undergo transplant, the 5-year survival is approximately 65% to 70%; the 5-year recurrence rate is less than 20%. The 5-year survival rate following liver resection (governed by a different set of operative criteria) is about equal to that following transplant. This result is in spite of a majority of patients experiencing metastases or a recurrence in the remaining part of the liver. Fewer than 20% of resected patients who have tumor recurrence are able to undergo transplant. Seventy-five percent of patients have unresectable disease that is diagnosed at an advanced stage. Median survival in these patients can range from months to years, depending on the stage of disease, the biologic aggressiveness of the tumor, vascular invasion, and “background” liver function, among other important factors.
Etiology and Risk Factors
Hepatitis B
The annual incidence of hepatocellular carcinoma in HBV carriers is 0.5%, and in patients with known cirrhosis, it is 2.5%. In one study, the relative risk of hepatocellular carcinoma in HBV carriers was 100 in Asian patients, who likely acquired the virus at birth. The epidemiology of HBV infection is different in the Western world, where it is acquired later in life, and risk in white individuals appears to be related to inflammatory activity and cirrhosis.
In areas in which hepatitis B is endemic, approximately 90% of all patients with hepatocellular carcinoma are positive for hepatitis B surface antigen (HBsAg). The presence of the hepatitis B “e” antigen has been found to increase risk nine-fold. An important reason to provide antiretroviral agents to patients with chronic HBV infection is the relationship between the risk of hepatocellular carcinoma development and absolute serum levels of HBV DNA. The most compelling epidemiologic evidence of a causal relationship between HBV infection and hepatocellular carcinoma is the observation of a significant decline in the incidence of childhood hepatocellular carcinoma after the introduction of a national immunization program in Taiwan. The hepatitis B “X” gene, which can interact with p53, has been a focus of study on the pathogenesis of hepatocellular carcinoma.
In a study following patients with chronic liver disease for development of hepatocellular carcinoma, 18,000 Chinese patients with chronic HBV infection were screened with α-fetoprotein determinations and liver ultrasonography every 6 months or received no screening for 5 years. Zhang et al found that the hepatocellular carcinoma mortality rate was 37% lower in the screened group than in the controls, and this outcome can be attributed to early-stage tumor detection and resection.
Hepatitis C
The risk of hepatocellular carcinoma in patients with chronic HCV infection and established cirrhosis is 2% to 8% per year. The molecular mechanisms of HCV infection and carcinogenesis are poorly understood, yet the disease is believed to occur in the context of chronic inflammation, which leads to fibrosis and cirrhosis. Unlike patients with HBV infection, patients with hepatocellular carcinoma infected with HCV usually have cirrhotic livers at diagnosis; this finding suggests an extended period of infection (or hepatic damage) before malignancy develops.
Alcohol
Patients with alcoholic cirrhosis are at risk for hepatocellular carcinoma, and the addition of HCV infection increases that risk dramatically. About 60 to 80 g of alcohol (equivalent to four to six beers) must be consumed daily over 5 to 10 years for a male to develop cirrhosis (odds ratio = 4). The interaction between alcohol consumption and HCV infection approximately doubles the odds ratio.
Other possible etiologies
These include aflatoxin, hemochromatosis, hepatic venous obstruction, Thorotrast (a contrast agent no longer used for radiologic procedures), androgens, estrogens, and α1-antitrypsin deficiency. Emerging risk factors for hepatocellular carcinoma are obesity and nonalcoholic fatty liver disease. In a prospective, cohort study of patients with biopsy-proven nonalcoholic steatohepatitis, older age and advanced fibrosis were risk factors for hepatocellular carcinoma.
Prevention
A large 5-year randomized study performed in China has confirmed that treatment of chronic HBV infection with the antiviral lamivudine(Epivir) not only decreases the risk of progression to cirrhosis of the liver but also decreases the rate of progression to hepatocellular cancer. Treated patients had a 3.9% risk of hepatocellular cancer over 5 years, compared with 7.4% for those in the placebo group (hazard ratio [HR] = 0.49; P = .047). It remains controversial whether antiviral therapy with ribavirin
(Copegus, Rebetol) and interferon decreases the risk of hepatocellular carcinoma in cirrhotic patients with HCV infection who achieve a sustained virologic response. If there is any benefit to therapy in such cases, it appears to be small.
Signs and Symptoms
Nonspecific symptoms
Patients with hepatocellular cancer usually present with abdominal pain and other vague symptoms, including malaise, fever, chills, anorexia, weight loss, and jaundice.
Physical findings
An abdominal mass is noted on physical examination in one-third of patients with hepatocellular cancer. Less common findings include splenomegaly, ascites, abdominal tenderness, muscle wasting, and spider nevi. Up to 10% of patients may present with an acute abdomen due to a ruptured tumor.
Screening and Diagnosis
Presently, no organization recommends routine screening of average-risk, asymptomatic adults for liver, gallbladder, and biliary tract cancers.
α-Fetoprotein
This serum marker is produced by 70% of hepatocellular carcinomas. The normal range for this serum marker is 0 to 20 ng/mL, and a level greater than 200 ng/mL is essentially diagnostic for hepatocellular cancer in the absence of chronic, active HBV infection. In the presence of active hepatitis B infection, the diagnostic cutoff is considered to be at least 1,000 ng/mL. In the setting of HCV infection, the cutoff for diagnosis of hepatocellular carcinoma has not been well studied. In hepatitis C, values greater than 200 ng/mL appear to be highly predictive of hepatocellular carcinoma. False-positive results may be due to acute or chronic hepatitis, germ-cell tumors, or pregnancy. In its guidelines for diagnosis of hepatic masses suspicious for hepatocellular carcinomas, the National Comprehensive Cancer Network does not recommend biopsy for patients with an α-fetoprotein level greater than 400 ng/mL, except in the presence of HBsAg positivity. In that situation, a cutoff value of 4,000 ng/mL is recommended for biopsy.
Hepatitis B and C
Given the association between hepatitis B and C and hepatocellular cancer at the time of diagnosis of a hepatic mass, blood should be sent for hepatitis B and C antigen and antibody determinations.
Imaging
The initial diagnostic test in the symptomatic patient may be ultrasonography, because it is noninvasive and can detect lesions as small as 1 cm. Negative results on ultrasonographic examination should not be accepted as diagnostic. Ultrasonographic findings should be followed up with more specific imaging.
Triple-phase, high-resolution computed tomography (CT) and contrast-enhanced magnetic resonance imaging (MRI) are the primary imaging modalities used to diagnose and stage hepatocellular carcinoma. Typically, hepatocellular carcinoma is a hypervascular tumor in the arterial phase with washout in the portal venous phase. Reports have documented a high number of false-positive results with CT angioportography and CT hepatic angiography. Lesions that meet the arterial “blush” criteria in the patient at high risk for hepatocellular carcinoma may not require biopsy for confirmation of cancer. This is particularly true for patients with elevated α-fetoprotein levels. CT predicts resectability in only 40% to 50% of cases and does not accurately determine the functional extent of cirrhosis. Major difficulties arise when the liver parenchyma is not homogeneous and the lesions are smaller than 1 cm. Use of positron emission tomography scanning is not defined in this disease, and the uptake of 18F-fluorodeoxyglucose by hepatocellular tumors is variable.
Laparoscopy
This procedure is useful for the evaluation of small tumors, the extent of cirrhosis, peritoneal seeding, guidance during biopsy and ablation of tumors, and the assessment of the volume of noninvolved liver. Although CT and MRI provide excellent preoperative staging, they may be used before open laparotomy. Laparoscopic or intraoperative ultrasonography should be used to confirm preoperative imaging tests. The laparoscopic ultrasound results may change surgical management in up to one-third of select patients.
High-risk patients
Individuals at high risk should be screened for hepatocellular carcinoma using ultrasonography and serum α-fetoprotein levels. When ultrasonograms are difficult to interpret, contrast-enhanced CT or MRI may be considered. Screening increases the proportion of cancers that are resectable. However, a study comparing 6-month and 12-month survival intervals in a cohort of HCV-infected patients with hemophilia showed no substantial benefit of more frequent screening.
Pathology
Three morphologic patterns of hepatocellular carcinoma have been described: nodular, diffuse, and massive. Diffuse and massive types account for more than 90% of cases. The nodular type usually has multiple lesions in both lobes.
Histologic arrangements
Several histologic arrangements have been identified: trabecular; compact; pseudoglandular or acinar; clear cell; and a fibrolamellar variant, which is associated with a relatively favorable prognosis and a younger age at diagnosis. The fibrolamellar variant is more commonly resectable and is not usually associated with infection and cirrhosis.
Staging and Prognosis
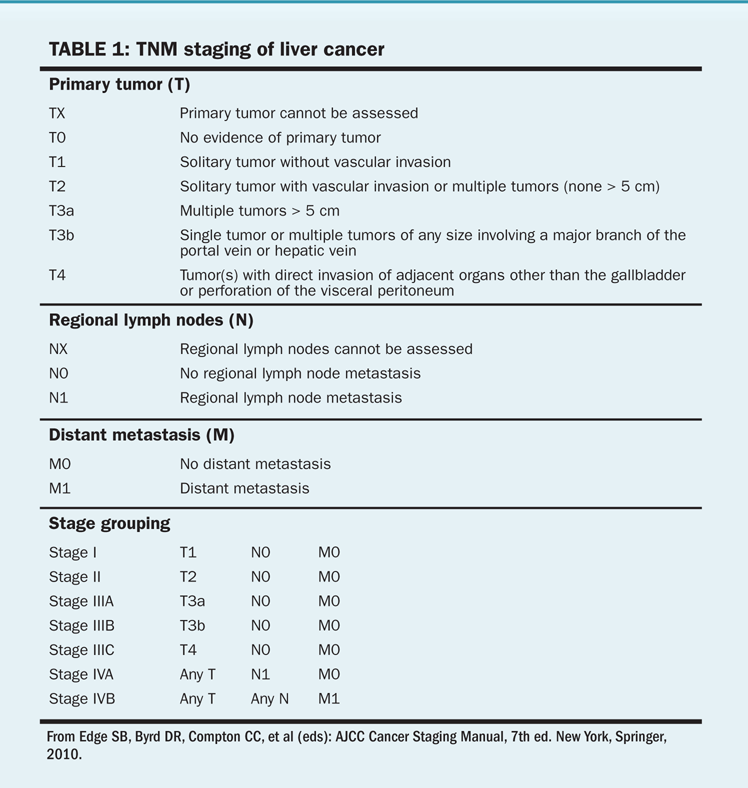
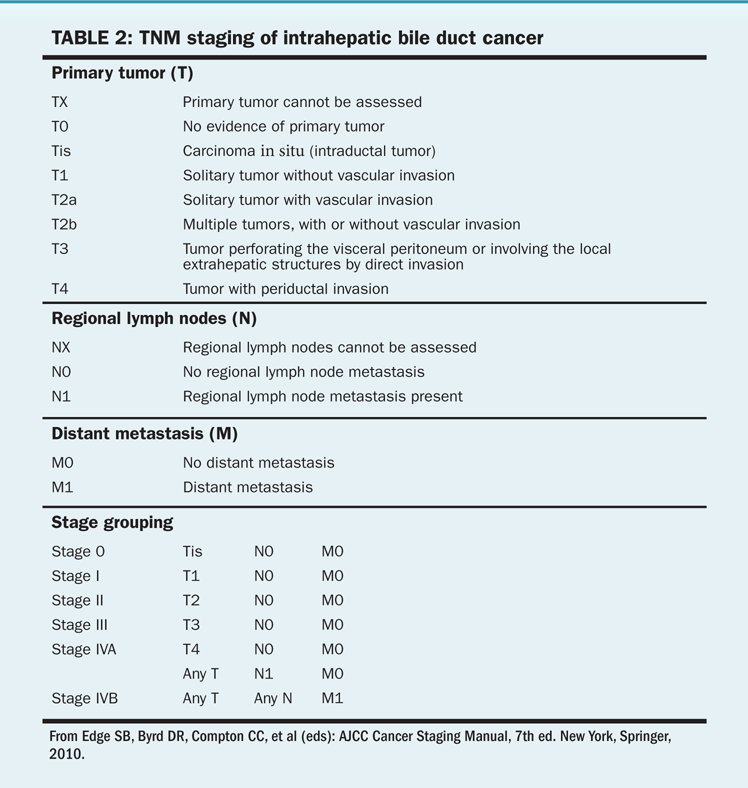
The staging system for hepatocellular cancer is based on the number and size of lesions and the presence or absence of vascular tumor invasion (Tables 1 and 2). The Okuda staging system accounts for the degree of liver dysfunction and may better predict prognosis than the TNM staging system. However, the Okuda staging system does not adequately predict resectability and primarily predicts end-stage disease. The Child-Pugh system and MELD (Model for End-Stage Liver Disease) scores measure liver function and are not cancer staging systems. Because of the limited value of standard staging, the most important factors that determine survival are technical resectability of lesions and the degree of dysfunction of the normal liver. Groups in Spain, Italy, and China have created prognostic indices that may prove useful for making treatment decisions. The Barcelona Clinic Liver Cancer staging system was designed to be a diagnostic and treatment strategy to compare tumor stage, liver function status, and performance status in its schema. Surgical resection is considered to be the best treatment for patients with solitary tumors and normal bilirubin levels who do not have portal hypertension. According to the Barcelona schema, patients may be considered for liver transplant if the above criteria are not met or for ablation if the disease is at an early stage (solitary tumors < 5 cm or up to three nodules with no single nodule > 3 cm). Such patients will have a 5-year survival of 50% to 75%. Chemoembolization is appropriate for patients with intermediate-stage disease who are asymptomatic and have preserved liver function, with a bilirubin level of less than 3 mg/dL; their 3-year survival will be 50% or better. For patients with advanced disease, there are fewer established therapeutic options; enrollment in a research study may be the best of these alternatives.
Of the roughly 20% of patients who can undergo resection, factors associated with improved survival include curative resection, small tumor size, well-differentiated tumors, and normal performance status. Cirrhosis, nodal metastases, and an elevated prothrombin time are indicative of a poor prognosis, as are male sex, age over 50 years, poor performance status, duration of symptoms < 3 months, tumor rupture, aneuploidy, high DNA synthesis rate, hypocalcemia, vascular invasion, and a high serum α-fetoprotein level.
Treatment
Surgery
Surgery offers the greatest potential for cure, albeit for a small number of patients with this diagnosis. Careful patient selection requires complete preoperative staging.
Only stage I or II tumors have a significant likelihood of being resectable for cure. Resectability is limited by the functioning liver tissue at the completion of a negative margin (R0) operation. Therefore, even a large tumor may still be potentially resectable for cure. Moreover, contiguous involvement of large vessels (including the portal vein and inferior vena cava) or bile ducts does not automatically mitigate against a resection. Resection is contraindicated in patients with metastatic disease to non-portal nodes and in extrahepatic locations. The use of the Child-Pugh score and volumetric evaluation aids in assessment of resectability. Other evaluations of liver functional and structural status, such as indocyanine green excretion and portal wedge pressure, are used infrequently to predict post-resection liver failure. For cirrhotic patients in whom less than 30% to 35% of the liver remains at the completion of resection, operative treatment is contraindicated. Likewise, this is true for noncirrhotic patients in whom less than 25% to 30% of the liver remains.
Bilobar disease may be addressed with formal resection, tumor ablation techniques (eg, radiofrequency ablation [RFA], microwave ablation, cryotherapy, and ethanol injection ablation), or a combination of the modalities.
Contraindications to resection. These include factors related to the medical comorbidities rendering the patient a nonoperative (nonsurgical) candidate: uncorrectable clinical hepatic failure (jaundice in the absence of biliary obstruction), hypoalbuminemia, ascites, renal insufficiency, hypoglycemia, prolongation of the prothrombin and partial thromboplastin times. Main portal vein involvement, extrahepatic metastatic disease, or the requirement for an R0 resection and/or ablation that would leave inadequate hepatic reserve would preclude surgery of any kind. These parameters are for the most part included in the Childs-Pugh classification and augmented with the other elements described.
TABLE 3 Child-Pugh score
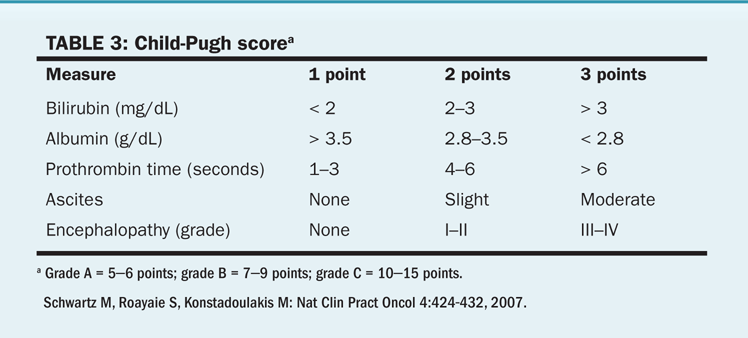
Noncirrhotic vs cirrhotic patients. For lesions 3 cm and smaller, RFA done either open or laparoscopic-assisted is considered to be the primary treatment for resectable hepatocellular carcinoma. Resection of hepatocellular carcinoma in the presence of cirrhosis is associated with increased morbidity. Cirrhosis had been a major deterrent to resection in Western nations. Historically, resectability rates varied from 0% to 43% for cirrhotic patients, whereas up to 60% of patients without cirrhosis had resection. This bias is being addressed with better preoperative functional evaluation and use of minimally invasive surgical techniques. Use of the modified Child-Pugh classification of liver reserve (Table 3) may guide the surgeon in preoperative assessment of liver function status and may aid in the selection of operable patients.
When resection is performed in the presence of cirrhosis, Child-Pugh class A patients fare better than do Child-Pugh class B or C patients. Survival rates at 5 years following resection range from 4% to 36%, with noncirrhotic patients living longer than cirrhotic patients. However, this survival is a combination of the morbidity of the chronic liver disease and higher risk of new primary hepatocellular cancer.
Transplant. Transplant has become an option for patients with hepatocellular cancer and cirrhosis. The transplant addresses the malignancy as well as the hepatic dysfunction. Therefore, it is a particularly excellent option when these two conditions occur synchronously. In addition, the distribution of grafts to this patient population fulfills both goals. A study of 181 patients with hepatocellular carcinoma (Iwatsuki et al: Ann Surg 1991) found similar overall 5-year survival rates in patients treated with transplant vs resection (36% vs 33%). Survival rates were similar in the two groups when tumors were compared for TNM stage. However, survival was significantly improved in patients with concomitant cirrhosis if they were treated with transplant. Tumor recurrence rates for stages II and III tumors were significantly lower after transplant than after resection, but no differences were seen for stage IV tumors. The cause of the cirrhosis may affect the transplant success. For patients with hepatitis B– or hepatitis C–associated hepatocellular carcinoma, the viral cause and status of the viral load are important considerations. Hepatitis C patients have a higher risk of hepatocellular carcinoma recurrence, lower graft survival, and increased mortality. Numerous additional studies have examined the relative value of transplant, ablation, and resection. There is much debate over their interpretation owing primarily to the selection of patients.
Therapies for nonresectable hepatocellular carcinoma
Given the high risk of recurrence after resection, the multifocal nature of hepatocellular carcinoma, and its association with chronic liver disease, therapies for nonresectable disease in patients who cannot withstand an operation or minimal resection can play an important role in management. A number of prognostic factors have been identified for patients with unresectable hepatocellular carcinoma. These factors, taken alone, can greatly affect survival rates, making cross-treatment comparisons more difficult because considerable selection bias may be present in any nonrandomized trial. In addition, direct comparisons are difficult, because there are few randomized trials among the large number of nonresectional, liver-directed therapies currently available. It is worth noting that a number of these treatments do not have overlapping toxicities and that research into combinations is potentially worthwhile.
Ablation
Ablation techniques include RFA, microwave ablation, cryotherapy, and injection of chemicals (eg, ethanol) directly into the tumor. RFA can be performed via laparotomy, laparoscopic-assisted with ultrasound guidance, or percutaneously with CT or ultrasonographic guidance. It is best suited for lesions smaller than 3 cm. The overall recurrence rate after ablation is related to the intent—cure vs control, clinical status, and technique. Some recurrence represents undertreatment of lesions, microscopic satellites that were not included in the ablation site, poor imaging due to technical issues, inexperience of the ablator, and selection of large tumors. Ablation is safe and effective for patients who cannot undergo resection or who need a bridge to transplant.
Adverse effects of RFA may include pleural effusions and peritoneal bleeding. In 1,000 patients treated with RFA for hepatocellular carcinoma, neoplastic seeding from percutaneous RFA occurred at a rate of 3.2% per patient, or 1.8% per treatment. The patients underwent 1,845 RFA treatments for 3,837 nodules; 20% had nodules larger than 3 cm. The observation period was 5 to 64 months, and poor differentiation was the only risk factor for neoplastic seeding in multivariate analysis. Surrogate markers for poor differentiation were larger tumor size and elevated tumor marker levels. Other investigators have reported occurrences of “seeding” in as many as 12% and as few as 0.9% of patients undergoing RFA for hepatocellular carcinoma, with subcapsular tumor location being a risk factor. A Japanese study by Morimoto et al compared the efficacy of RFA combined with transarterial chemoembolization (TACE) vs RFA alone in patients with intermediate-sized hepatocellular carcinoma (3.1 to 5 cm) in a small, randomized, controlled trial. The rate of local tumor progression was 39% in the RFA-only group vs 6% in the TACE-RFA combination group at the end of 3 years. However, there was no statistical difference in the overall survival rates between the two groups. TACE before RFA may be beneficial because it enables better ablation and possibly facilitates the treatment of larger hepatocellular carcinomas.
• Intratumoral ethanol injection—The direct injection of 95% ethanol into a neoplastic lesion causes cellular dehydration and coagulation necrosis. This procedure has been largely replaced by RFA, which in several clinical trials has shown efficacy superior to that of ethanol injection.
Cryotherapy, Microwave Ablation, and RFA
These techniques require image guidance to ensure accurate device placement and assessment of extent of ablation during the active phase of treatment (tissue destruction). Cryotherapy (largely replaced by RFA) and RFA techniques are suitable for treating localized disease. Cryotherapy has been used intraoperatively to ablate small, solitary tumors outside a planned resection (ie, in patients with bilobar disease). Cryotherapy must be performed using laparotomy. In performing RFA and microwave ablation, the wires and antennae, respectively, are placed within the tumor. For RFA, the current creates ionic agitation and heat, which causes coagulative necrosis at the site of the tumor and to a margin in the periphery. Microwave ablation uses microwaves of specific intensity and duration to create heat for tissue destruction. Average temperatures generated are in the 100°C to 110°C range. RFA has efficacy superior to that of ethanol injections for lesions larger than 2 cm. Posttreatment imaging is performed 6 to 12 weeks postoperatively and should evaluate the extent of tumor destruction and local peripheral failures. The imaging report will paradoxically describe a larger lesion because of the destruction of both the primary hepatocellular carcinoma and a margin around it creating an R0 ablation.
Hepatic transcatheter embolization (TACE). Normal hepatocytes receive most of their blood supply from the portal vein, whereas tumors create new blood vessels from branches of the hepatic arterial system. This target is exploited by embolization of the hepatic artery with any number of substances, resulting in radiographic responses in about 50% of patients and evidence of tumor liquefaction in more than two-thirds of patients. At 2 years, the overall survival benefit from this procedure ranges from 20% to 60%. Embolization is accomplished by advancing a catheter within the tumor-feeding branch of the hepatic artery. Materials injected have included polyvinyl alcohol, iodized oil (Lipiodol), collagen, and autologous blood clot. If chemotherapy is given, it usually is suspended in iodized oil, which is retained in the tumor. Common chemotherapeutic agents used have included cisplatin and doxorubicin(Drug information on doxorubicin), which may produce systemic adverse effects. Postembolization syndrome, with fever, abdominal pain, and, occasionally, ileus, occurs in 50% of patients when a large volume of liver is treated. Complications include infections and abscesses. Because few complete responses are achieved, treatments may be repeated on the same tumor for better results. Because of the risk of liver failure, TACE may be contraindicated in patients with portal vein thrombosis and for most individuals with Child-Pugh B status. Patients with Child-Pugh C liver disease cannot receive chemoembolization because of the risk of liver failure. One innovation in chemotherapy delivery during TACE is the use of drug-eluting beads (DEBs). These perforated beads allow for slow, localized release of chemotherapy after embolization. In a study by Varela et al, 27 patients with Child-Pugh A liver disease and unresectable hepatocellular carcinoma underwent DEB-TACE. Pharmacokinetic samples were analyzed for doxorubicin, demonstrating lower systemic drug exposure than expected with conventional TACE. Two patients had abcesses, but radiographic response rate was a promising 75%. Studies of this technology are ongoing.
Sidebar: A prospective, single-center, phase II study investigated combination therapy with sorafeniband DEB-TACE in patients with unresectable hepatocellular carcinoma Sorafenib was an attractive agent to use because of the elevations in vascular endothelial growth factor and platelet-derived growth factor levels that occur after TACE procedures. Thirty-five patients with advanced hepatocellular carcinoma and Childs-Pugh A or B liver dysfunction were entered into the study and sorafenib 400 mg twice daily was started one week before DEB-TACE and continuously thereafter. The study demonstrated a disease control rate of 95% based on RECIST criteria (9% partial response and 86% stable disease). Furthermore, the toxicity profile of sorafenib plus DEB-TACE appears to be similar to that of sorafenib alone. The most common adverse effects included fatigue (50%), hand-foot-skin reaction (30%), and right upper quadrant pain (18%), with most toxicities being grade 1 to 2 (Pawlik TM et al: J Clin Oncol 29:3960-3967, 2011). SPACE (Sorafenib or Placebo in Combination with TACE) was a large, randomized, phase II trial (307 patients) that randomized patients to sorafenib 400 mg twice daily or placebo with DEB-TACE. The primary endpoint was time to progression and overall survival was a secondary endpoint. For the sorafenib arm, the HR for time to progression was 0.797 (95% confidence interval [CI], 0.588–1.08; P = .072) and the HR for overall survival was 0.898 (95% CI, 0.606–1.330; P = .295). The authors concluded that the study met its primary endpoint of improving time to progression. However, the study was unusual in that a P value of .15 was considered significant on the basis of the trial’s design. Overall survival data were immature. These results do not suggest a large benefit of sorafenib in this setting, but do support the continuation of more definitive studies, such as the ongoing ECOG-1208 trial (TACE with sorafenib vs TACE alone; Lencioni R et al: J Clin Oncol 30[4s] abstract LBA154, 2012).
Radiation therapy.
• Adjuvant treatment—There is no evidence that adjuvant radiation therapy can improve local or regional tumor control after adequate resection or ablation.
• Unresectable disease—Whole-liver radiation therapy can provide palliation in patients with unresectable tumors but is limited to a total dose of 30 Gy or less because of the risk of radiation-induced liver disease. Whole-liver irradiation has been combined with chemotherapy and TACE, with objective response rates of approximately 40% to 50% and median survival rates of about 18 months. Patients with tumor regrowth after chemoembolization may respond to radiation therapy. Radiation therapy has also been delivered using 90yttrium microspheres (radioembolization) infused via the hepatic artery to carefully selected patients; response rates as high as 89% have been reported. One study reported that 56% of treated T3 tumors were downstaged to T2, which allowed for subsequent liver-directed therapy or use as a bridge to transplant. Randomized trials of liver-directed therapy are uncommon. In a retrospective comparison of a single institution’s experience with chemoembolization compared with radioembolization, Salem et al found there was a benefit in time-to-progression (13.3 months vs 8.4 months; P = .046) for radioembolization in 245 patients, although there was no difference in overall survival. A report by Sangro et al of the results of treatment with radioembolization in 325 patients at eight European centers found an overall median survival of 13 months (24.4 months in Barcelona Clinic Liver Cancer stage A).
Three-dimensional conformal radiation therapy planning can allow patients with nondiffuse disease to be safely irradiated to doses well above the whole-liver tolerance dose, with doses up to 90 Gy given safely to select patients, resulting in a response rate of 40% and a median survival of 15 months. Conformal radiation therapy has been successfully combined with TACE in patients with portal vein tumor thrombosis, using the conformal radiation therapy to target the portal vein thrombus and TACE to treat the rest of the liver. In a study of more than 400 patients treated with this combination, the median survival was 10.6 months with a 2-year survival rate of 23%.
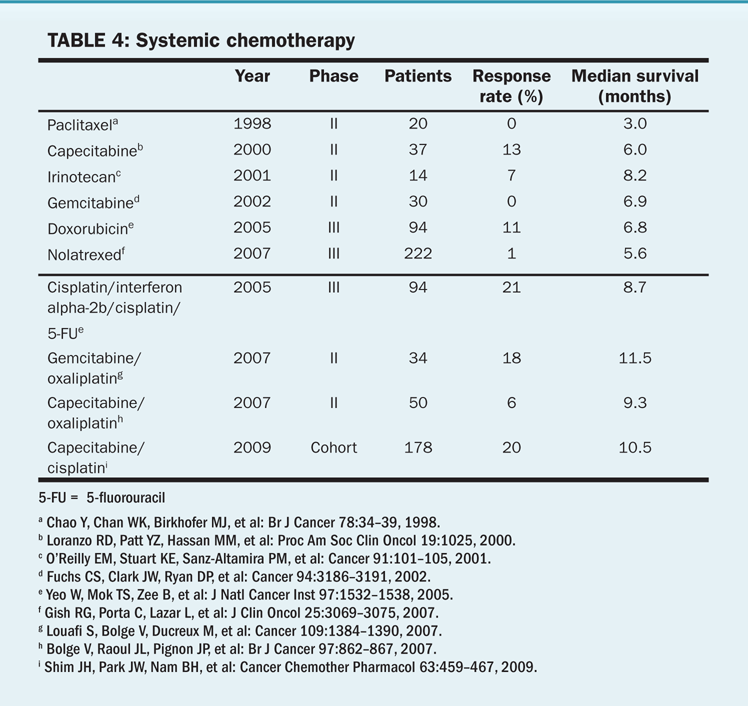
TABLE 4 Systemic chemotherapy
Multiple institutions have reported response rates as high as 90%, with acceptable toxicity when conformal radiation therapy was combined with TACE. Good response and local tumor control rates have also been reported for proton, carbon ion, and stereotactic radiation therapy. In select patients, Andolino et al found stereotactic radiation therapy to have an excellent local control rate (90% at 2 years for tumors measuring 6 cm or less) and a median time-to-progression of 4 years.
Chemotherapy.
• Systemically administered chemotherapy—The low response rates to chemotherapy are related to the role of the hepatocyte in detoxification. Hepatocytes also have high multidrug resistance expression. Furthermore, many patients with hepatocellular carcinoma have cirrhosis or hepatic dysfunction, which complicates the administration of chemotherapeutic agents that undergo hepatic metabolism. Agents with partial response rates near or above 10% include doxorubicin, 5-fluorouracil (5-FU), and cisplatin. Table 4 describes the results of studies testing various chemotherapeutic agents in patients with hepatocellular carcinoma.
A double-blind phase II trial by Abou-Alfa et al was done in Child-Pugh A patients with advanced hepatocellular carcinoma. Doxorubicin combined with sorafenib was compared with doxorubicin alone. Median overall survival was 13.7 months in the combination-therapy arm vs 6.5 months in the doxorubicin-only arm. The progression-free survival was 6 months vs 2.7 months for these groups, respectively. The toxicity profile was similar to those of individual single agents. The combination of doxorubicin with sorafenib showed favorable results compared with doxorubicin alone. This is the basis for an ongoing phase III trial (CALGB 80802) of doxorubin with sorafenib compared with sorafenib alone.
Biologic therapy
Inhibiting angiogenesis in hepatocellular carcinoma is based on several factors. Hepatocellular carcinoma is a vascular tumor; increased levels of vascular endothelial growth factor are found in hepatomas compared with normal hepatic tissue, and increased endothelial growth factor levels before resection of tumor or TACE are associated with early relapse, aggressive behavior, and poor prognosis. Other targets include epidermal growth factor receptor (EGFR, which is frequently expressed in hepatoma cells), the mitogen-activated protein kinase (MAPK) pathway, and the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. Conventional markers of radiographic response are poorly related to tumor cell kill in hepatocellular carcinoma. More meaningful markers of therapeutic benefit may be time to disease progression, progression-free survival, and overall survival.
Erlotinib (Tarceva), an oral agent that inhibits the EGFR-related tyrosine kinase enzyme, was evaluated for efficacy in hepatocellular carcinoma. In a number of phase II trials, agents targeting EGFR have been tested in patients with hepatocellular carcinoma, and they appear to have only modest activity. In two phase II studies with erlotinib, “disease control” was greater than 50% (response rate > 10%), and median survival was 10 to 13 months. The combination of bevacizumab (Avastin) and erlotinib in a phase II study of 40 patients with Child-Pugh A or B liver disease that was not amenable to surgery or regional therapy resulted in a progression-free survival of 9 months and a median survival of 15.6 months, which compares favorably with sorafenib. Use of this regimen in the cirrhotic patient may be associated with bleeding and thrombosis.
The SHARP (Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol) trial compared sorafenib (Nexavar, 400 mg PO bid) with placebo in 600 patients with Child-Pugh class A liver disease. About one-third of patients had undergone embolization, and the disease progressed. In all, 70% of patients had portal vein thrombosis and/or metastatic lesions. In spite of a low radiographic response rate, median survival for the 300 patients given sorafenib was significantly better than that for the placebo group (10.7 months vs 7.9 months; HR = 0.69). This is the first well-powered trial to convincingly demonstrate a survival benefit with the use of a systemic agent for this disease. Sorafenib is the standard of care for hepatocellular carcinoma not amenable to locoregional therapy and is the first FDA-approved agent for this indication. Studies using the drug with embolization are ongoing. In the Asia-Pacific Trial, 226 patients with Child-Pugh class A liver disease were randomized to receive sorafenib or placebo. These patients primarily had HBV infection; compared with the SHARP study patients, they had more local therapy before their entry into the trial and, as a whole, more advanced disease. Median survival was 6.5 months, compared with 4.2 months for sorafenib and placebo (HR, 0.68). More data are needed to establish the role of sorafenib in treating Child-Pugh class B liver disease.
The GIDEON registry (Marrero et al) is an ongoing, global, prospective study of patients with Child-Pugh A and Child-Pugh B hepatocellular carcinoma who are suitable for sorafenib therapy. The second interim analysis showed that treatment-related adverse effects were similar in both Child-Pugh A and Child-Pugh B patients but that treatment-related serious adverse events were more common in the Child-Pugh B patients. As a result, a greater percentage of the Child-Pugh B patients ultimately had to discontinue treatment (38% vs 23%). In the intent-to-treat population (1614 patients), preliminary median overall survival was 10.5 months in the Child-Pugh A group and 4.8 months in the Child-Pugh B group. This suggests that although it may be safe to use sorafenib in CP-B patients, close attention must be paid to the adverse effects and the benefit, if any, of sorafenib in this group.
Sidebar: The US data from the second interim analysis of the GIDEON study showed that the median sorafenib dosages in Child-Pugh A and Child-Pugh B patients were 580 mg/d and 577 mg/d, respectively. The median treatment duration was 14.4 weeks in Child-Pugh A patients and 10.1 weeks in Child-Pugh B patients. Grade 3/4 treatment-related adverse events were 22% and 23% in Child-Pugh A and Child-Pugh B patients, respectively. Despite a similar toxicity profile and similar sorafenib dosing in both groups, Child-Pugh B patients were more likely to discontinue sorafenib because of adverse effects, resulting in a shorter treatment duration (Piperdi B et al: J Clin Oncol 30[4s]:abstract 282, 2012).
Sidebar: Brivanib is oral selective dual inhibitor of vascular endothelial growth factor (VEGF) receptor and fibroblast growth factor (FGF) receptor. The FGF pathway may play a role in the development of resistance to anti-VEGF therapy. Brivanib demonstrated antitumor activity in a phase II first-line study for the treatment of advanced hepatocellular carcinoma (Park JW et al: Clin Cancer Res 17:1973-1983, 2011). Therefore, a second-line phase II trial was conducted in patients with hepatocellular carcinoma who had progressed or had been intolerant of prior antiangiogenic treatments, and it showed promising results (Finn RS et al: Clin Cancer Res 18:2090-2098, 2012). Results from the phase III study (BRISK-PS) were recently presented in abstract form. The BRISK-PS study randomized 395 patients in a 2:1 fashion between brivanib 800 mg PO daily and placebo. Patients who entered the study were required to have been treated with sorafenib for at least 14 days and then progressed on or were intolerant of sorafenib. The primary endpoint was overall survival and key secondary endpoints were time to progression and disease control rates. Median overall survival was 9.4 months vs 8.2 months (HR = 0.89; CI, 0.69–1.15) in the brivanib-treated group vs the placebo group, respectively. Therefore, the primary endpoint for improved overall survival was not met. Median time to progression was 4.2 months vs 2.7 months (HR = 0.56; CI, 0.42–0.76) in the brivanib-treated group vs the placebo group, respectively. In spite of the study not meeting its primary endpoint, the improvment in time to progression suggests that brivanib does have some activity in this population (Llovet JM: EASL International Liver Congress 2012, Barcelona, Spain).
BILIARY TRACT CANCERS
Gallbladder carcinoma is diagnosed approximately 5,000 times a year in the United States, making it the most common biliary tract tumor and the fifth most common gastrointestinal tract cancer. Approximately 4,500 cases of bile duct tumors occur each year in the United States.
Epidemiology
Gallbladder cancer
Gender. Women are more commonly afflicted with gallbladder cancer than are men, with a female to male ratio of 1.7:1.
Age. The median age at presentation of gallbladder cancer is 73 years.
Race. An incidence five to six times that of the general population is seen in southwestern Native Americans, Hispanics, and Alaskans.
Bile duct cancer
Gender. Bile duct tumors are found in an equal number of men and women.
Age. Extrahepatic bile duct tumors occur primarily in older individuals; the median age at diagnosis is 70 years.
Etiology and Risk Factors
Gallbladder cancer
The risk of developing gallbladder cancer is higher in patients with cholelithiasis and calcified gallbladders and in typhoid carriers. Gallstones are present in 70% or more of patients with gallbladder cancer and presumably cause chronic inflammation. The overall incidence of gallbladder cancer in individuals with cholelithiasis is 1% to 3% and in patients with so-called porcelain gallbladders, caused by chronic cholecystitis, 10% to more than 50%. In patients who have gallbladder polyps measuring more than 1 cm, the risk of cancer is high.
Bile Duct Cancer
Primary sclerosing cholangitis (PSC). Thirty percent of cholangiocarcinomas are diagnosed in patients with PSC with or without ulcerative colitis. The annual incidence of cholangiocarcinoma in patients with PSC is estimated at 1.5% per year; their lifetime risk of developing this malignancy is 10% to 15%. These patients have a highly abnormal biliary system, making diagnosis of cholangiocarcinoma difficult.
Ulcerative colitis. The incidence of bile duct cancer in patients with ulcerative colitis is 9 to 21 times higher than that in the general population. This risk does not decline after total colectomy for ulcerative colitis.
Other risk factors. Congenital anomalies of the pancreaticobiliary tree, parasitic infections, biliary papillomatosis, and Lynch syndrome are also associated with bile duct tumors. No association of bile duct cancer with calculi, infection, or chronic obstruction has been found.
Signs and Symptoms
Gallbladder cancer
Early and late disease. In the early stages, gallbladder cancer is usually asymptomatic. Later, symptoms similar to those of benign gallbladder disease arise; they include right upper quadrant pain, nausea, vomiting, fatty food intolerance, anorexia, jaundice, and weight loss. This nonspecificity of symptoms delays presentation for medical attention and contributes to the low curability of gallbladder cancer.
Physical findings. These may include tenderness, an abdominal mass, hepatomegaly, jaundice, fever, and ascites.
Bile duct cancer
Jaundice. This is the most frequent symptom found in patients with high bile duct tumors; it is present in up to 98% of such patients.
Nonspecific signs and symptoms. Patients who do not present with jaundice have vague complaints, including abdominal pain, weight loss, pruritus, fever, and an abdominal mass.
Diagnosis
Gallbladder cancer
Gallbladder carcinomas are often diagnosed at an advanced stage, such that by the time symptoms have developed, most tumors are unresectable.
Laboratory values. Findings in patients with gallbladder carcinoma are nonspecific but may include anemia, leukocytosis, and an elevated bilirubin level.
Ultrasonography. This diagnostic study is useful for defining a thickened gallbladder wall and may show tumor extension into the liver. It is valuable in measuring the size of a polyp.
CT and MRI. CT is more helpful than ultrasonography in assessing adenopathy and spread of disease into the liver, porta hepatis, or adjacent structures. MRI may be used to evaluate intrahepatic spread.
Cholangiography. Endoscopic retrograde cholangiopancreatography (ERCP), transhepatic cholangiography (THC), or magnetic resonance cholangiography may be useful in the presence of jaundice to determine the location of biliary obstruction and involvement of the liver.
Bile duct cancer
Cholangiocarcinoma may present earlier than gallbladder cancer by virtue of the development of biliary obstruction with jaundice, which may be painless. Tissue confirmation of suspected bile duct cancer can be difficult. The goals of the diagnostic evaluation include the determination of the level and extent of obstruction, the extent of local invasion of disease, and the identification of metastases.
Many patients with cholangiocarcinoma are thought to have metastatic adenocarcinoma of an unknown primary site, although occasionally the metastatic lesion may produce biliary dilatation without the primary lesion itself being radiographically visualized. Recently, microarray-based technology for genetic analysis has become available to help characterize tumors that are difficult to identify.
Ultrasonography. It is generally accepted that ultrasonography should be the first imaging procedure in the evaluation of the jaundiced patient.
CT. This diagnostic modality is a complementary test to ultrasonography, but both tests are accurate for staging in only 50% of patients and for determining resectability in fewer than 45% of patients.
Cholangiography. This diagnostic technique is essential to determine the location and nature of the obstruction. Percutaneous THC is used for proximal lesions, and ERCP is used for distal lesions. Magnetic resonance cholangiopancreatography may replace invasive studies in the near future. Histologic confirmation of tumor can be made in 45% to 85% of patients with the use of exfoliative or brush cytology during cholangiography.
Pathology
Gallbladder cancer
Histologic types. More than 85% of gallbladder neoplasms are adenocarcinomas, and the remaining 15% are squamous cell or mixed tumors.
TABLE 5 TNM staging of gallbladder cancer
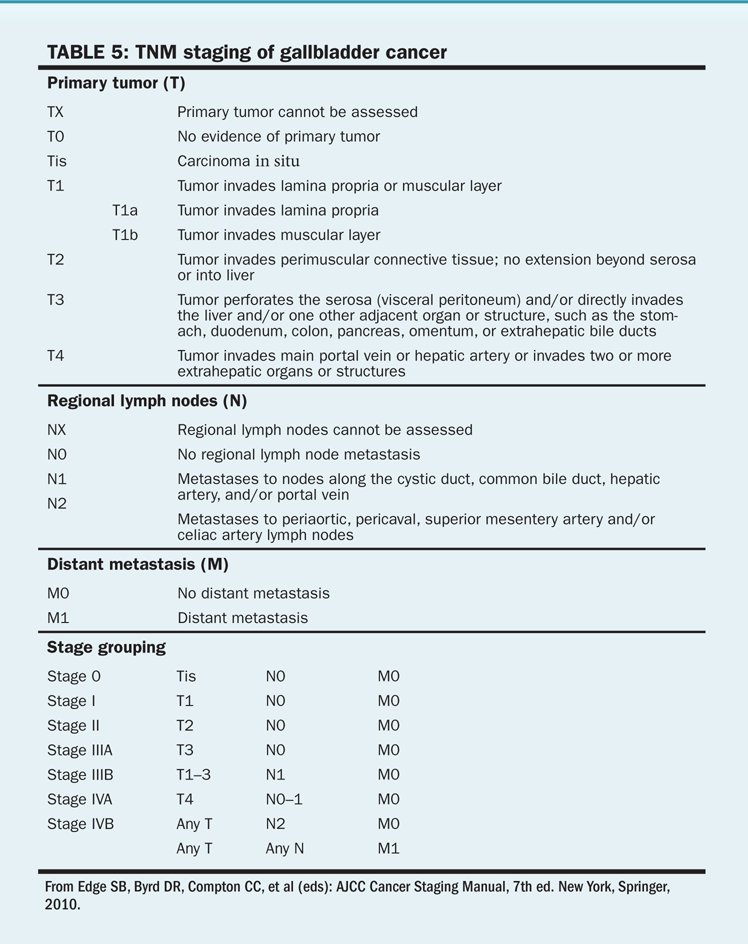
Route of spread. The initial route of spread of gallbladder cancer is locoregional rather than distant. For patients who undergo resection for presumed high-risk gallbladder masses or preoperatively defined disease limited to the gallbladder, 25% will have lymphatic involvement and 70% will have direct extension of disease into the liver defined at operation.
Bile duct cancer
Adenocarcinoma. Morphologically, more than 90% of bile duct tumors are adenocarcinomas. Three macroscopic appearances have been identified. The papillary and nodular types occur more frequently in the distal bile duct, whereas the sclerosing type is found in the proximal bile duct. Patients with papillary lesions have the best prognosis. Immunohistochemical staining may be positive for cytokeratin 7 and 20.
Other histologic types. Unusual malignant diseases of the biliary tract include adenosquamous carcinoma, leiomyosarcoma, and mucoepidermoid carcinoma.
Route of spread. Most bile duct tumors grow slowly, spreading frequently by local extension and rarely by the hematogenous route. Nodal metastases are found in up to one-third of patients.
Staging and Prognosis
Gallbladder cancer
TABLE 6 Staging of perihilar bile duct tumors
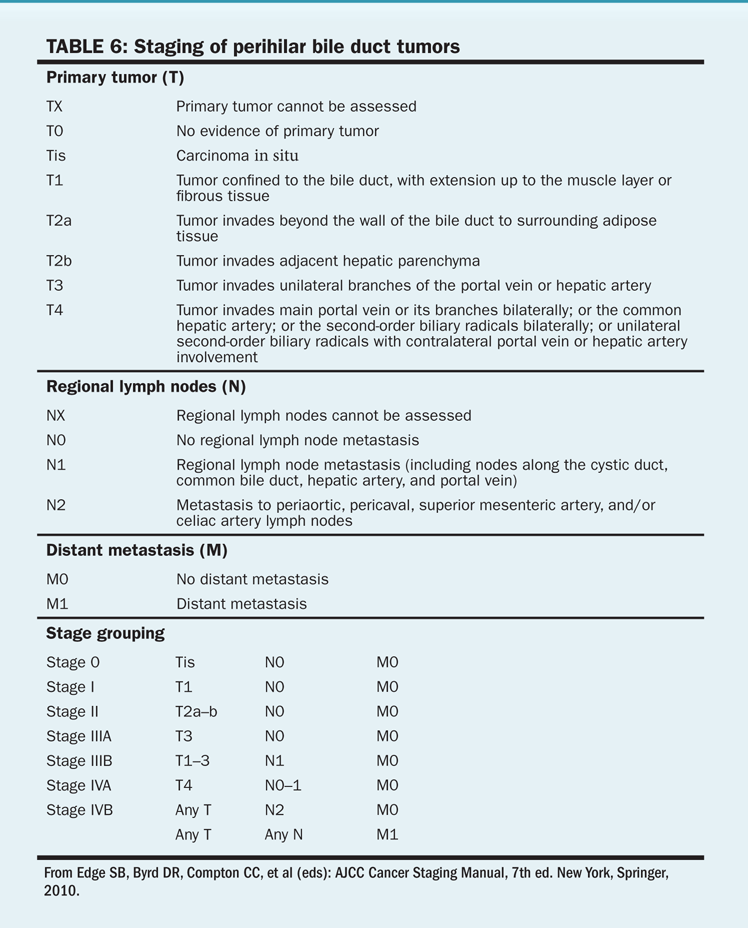
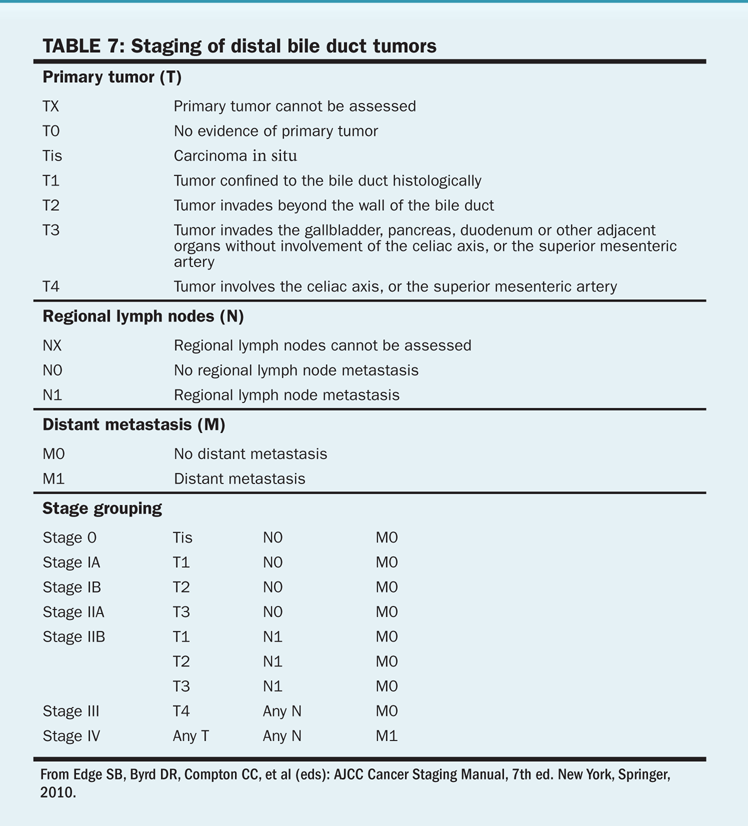
TABLE 7 Staging of distal bile duct tumors
Gallbladder cancer is staged primarily at the time of surgery, and staging is determined by lymphatic involvement and extension of disease into adjacent structures (Table 5).
Stage. Survival of gallbladder carcinoma is directly related to disease stage. The 5-year survival rate is 83% for persons whose tumors are confined to the gallbladder mucosa; this rate decreases to 33% if the tumor extends through the gallbladder. For patients who have involvement of the lymph nodes or metastatic disease, 5-year survival rates range from 0% to 15%.
Type of therapy. Median survival is also improved in patients who have undergone curative resection, compared with those who have had palliative procedures or no surgery (17 months vs 6 and 3 months, respectively).
Bile duct cancer
More than 70% of patients with cholangiocarcinoma present with local extension, lymph node involvement, or distant spread of disease. The American Joint Committee on Cancer (AJCC) staging system for extrahepatic tumors is shown in Tables 6 and 7.
Stage. Survival for these patients is poor and is directly related to disease stage. Median survival time is 12 to 20 months for patients with disease limited to the bile ducts and 8 months or less when the disease has spread.
Tumor location. Survival is also related to tumor location, with patients who have distal lesions doing better than those with mid or proximal tumors.
Success of therapy. Curative resection and negative margins result in improved survival.
Treatment
In the absence of polyps identified ultrasonographically and confirmed by CT during the workup of suspected cholelithiasis, relatively few gallbladder cancers are diagnosed before surgery. Only 1% to 2% of cholecystectomy specimens are found to contain malignancy.
Surgery for gallbladder cancer
Surgical management of gallbladder carcinoma is based on the local extension of the tumor. Surgery may be curative, but fewer than 30% of patients are potentially resectable at time of diagnosis.
Early-stage disease. Tumors that invade the lamina propria may be treated by cholecystectomy only. This is usually the situation in a gallbladder cancer that is identified by the pathologist following a cholecystectomy for presumed stone disease. Laparoscopic cholecystectomy should not be performed if the diagnosis of gallbladder cancer is suspected preoperatively (eg, gallbladder polyp > 1 cm on preoperative imaging, thickened gallbladder wall without a history of gallstones or cholecystitis). All suspected gallbladder cancers, as well as those documented intraoperatively and those for which the pathology on cholecystectomy identifies disease greater than T1a, should be treated with a formal resection.
The resection should include the gallbladder bed (segments IVb and V) and a portahepatic, paraduodenal, and gastrohepatic ligament lymphadenectomy, as well as a bile duct resection if the disease extends to the cystic duct.
Disease that involves the gallbladder node is particularly curable and should be resected. Nodal disease beyond the pericholedochal (porta hepatis) nodes defines the surgically incurable patient.
The usual pattern of spread is locoregional, although lymphangitic metastasis can be observed for T3 and T4 disease. On the basis of retrospective data, some surgeons will perform radical surgery in T3 and T4 disease with intent to cure.
Surgery for bile duct cancer
The rate of resectability is 15% to 20% for high bile duct tumors and up to 70% for distal lesions. Guidelines for surgery include the absence of retropancreatic and paraceliac nodal metastasis, noncontiguous liver metastasis, major vascular invasion, or extrahepatic invasion of adjacent organs. Some surgeons will attempt en bloc resection with vascular reconstruction.
Assessing resectability. High-resolution CT or MRI with biliary reconstruction may be supplemented with hepatic arteriography, portal venography, or duplex imaging preoperatively to assess resectability.
Preoperative treatments. Preoperative stenting for patients with jaundice caused by obstruction is controversial. Many surgeons find that placement of a biliary stent may confound imaging and impede surgery. This must be weighed against the benefit of relieving an obstruction and preventing infection (particularly if the biliary tree has been manipulated in the diagnostic evaluation). Three randomized trials have shown no benefit to preoperative decompression of the biliary tree in patients with obstructive jaundice. Some authors advocate the preoperative placement of biliary stents (non-metal) to facilitate dissection of the hilus. This procedure should be performed close to the planned resection to reduce the risk of cholangitis and maintain the duct at its maximally dilated size.
Proximal tumors. Local excision is often possible for proximal lesions. Hepatic resection is indicated for high bile duct tumors. Resection is not indicated in situations in which a clear surgical margin cannot be obtained.
Mid-ductal and distal tumors. Mid-ductal lesions can often be removed by resection of the bile duct with associated portal lymphadenectomy. Distal or mid-ductal lesions that cannot be locally excised should be removed by pancreaticoduodenectomy.
Reconstruction techniques. Biliary-enteric continuity is usually reconstructed with a Roux-en-Y anastomosis to the hilum for high lesions and in a standard drainage pattern following pancreaticoduodenectomy.
Liver transplant. This procedure has been attempted for unresectable tumors, but early recurrence and poor survival have prevented the widespread application of this approach. Protocols for transplant for patients with localized, unresectable disease who respond to neoadjuvant chemoradiation therapy are ongoing and promising in the highly select population. No comparative studies of patients treated with tranplant compared with best oncologic care (chemotherapy and radiation) are available.
Surgical bypass. For patients found to have unresectable disease at surgical exploration, operative biliary bypass may be performed using a variety of techniques. Bypass results in excellent palliation and obviates the need for further intervention.
Adjuvant radiochemotherapy for gallbladder cancer
Because of the rarity of biliary cancers, there is a paucity of prospective data to guide our practices. In the United States, gallbladder cancer commonly is treated with 5-FU and radiation after resection. A randomized trial performed in Japan showed that treatment with 5-FU and mitomycin produced a survival benefit in patients with resected gallbladder cancers. These data came from a planned subset of a larger trial in which 112 patients with gallbladder cancer were randomized. At 5 years, 26% of chemotherapy-treated patients were alive, compared with 14% of those treated with surgery and observation alone. On the basis of these data, consideration of chemotherapy for these patients is warranted, but definitive conclusions require a larger randomized trial. Two analyses using the SEER database have concluded that adjuvant radiochemotherapy may have a survival benefit for node-positive gallbladder cancer. One of these studies also found a benefit for tumors that were T2+.
For advanced, unresectable gallbladder cancer, palliation of obstructive jaundice and pain should be the goal. Combined-modality chemoradiation therapy may benefit patients with advanced disease. Regarding chemotherapy, many regimens that use oxaliplatin (Eloxatin) and gemcitabine(Gemzar), as well as capecitabine (Xeloda) alone or in combination with cisplatin have been tried. Modest response rates are the norm, and the indolence or aggressiveness of the tumor primarily influences survival.
Adjuvant chemoradiotherapy for cholangiocarcinoma
Delivery of adjuvant chemoradiotherapy is common for patients who have had margin-negative or microscopically positive margin resections, with observational series providing supporting evidence of improved outcomes. Chemoradiotherapy with infusional 5-FU or capecitabine is also given in the setting of positive regional lymph nodes. A meta-analysis by Horgan et al of 20 studies including more than 6,700 patients with either gallbladder or bile duct cancers concluded that adjuvant therapy with chemotherapy or chemoradiotherapy was superior to radiotherapy alone, with the greatest benefit in those with lymph node–positive and R1 disease.
Management of unresectable, locally advanced disease
The majority of patients present with unresectable disease, and palliative care is the goal of their management. Most of these patients experience a rapid decline in health. However, several chemotherapy regimens have shown promise for lengthening survival and are detailed in the Chemotherapy section.
There is considerable experience using brachytherapy alone or combined with external-beam radiation therapy for unresectable bile duct tumors. Median survival ranges from 10 to 24 months, and 5-year survival rates are approximately 10% with these approaches. Cholangitis, however, may occur more frequently in patients treated with brachytherapy.
Photodynamic therapy (PDT) in cholangiocarcinoma is an interesting option that may provide a significant survival benefit in addition to treating biliary stenosis and cholestasis and improving quality of life. A randomized trial of stenting alone vs stenting with PDT found a significantly longer median survival and improved quality of life in the PDT group. Although it is unlikely that all of the tumor was treated with PDT, this study did show the importance of local tumor control and its association with quality of life. As previously discussed, another palliative option is use of 90yttrium microspheres.
Chemotherapy
Biliary tract malignancies are uncommon, and the numbers of clinical trials and of patients in those trials are limited. Generally speaking, responses to chemotherapy are infrequent and brief. However, newer drugs and drug combinations are better tolerated and stand to improve on past results.
5-FU. This drug has historically been the most active single agent, with single-agent response rates in the 10% to 20% range.
Capecitabine. This prodrug of 5-FU (see “Colon, Rectal, and Anal Cancers” chapter) produced responses in 4 of 8 cases of gallbladder cancer but in only 1 of 18 cholangiocarcinomas in a phase II study presented by Hassan et al from the MD Anderson Cancer Center.
Gemcitabine. Multiple studies have documented gemcitabine as an active agent, particularly in gallbladder cancer.
Combination chemotherapy. Combined 5-FU and gemcitabine has modest activity in biliary malignancies. A group in Toronto published results of combination therapy with capecitabine and gemcitabine. The mix of cholangiocarcinoma and gallbladder cancer in the 45 patients studied was nearly 50-50. The overall response rate was 31%, and the median survival was a promising 14 months.
Valle et al followed up on the promising results of their phase II study with a randomized phase III study that compared cisplatin and gemcitabine combined with gemcitabine alone in patients with advanced biliary tract cancer. The median overall survival was 11.7 months in the cisplatin-gemcitabine group and 8.1 months in the gemcitabine-alone group. The median progression-free survival was 8 months in the combination group and 5 months in the gemcitabine-alone group. Adverse events were similar in both arms; however, neutropenia was more common in the cisplatin-gemcitabine group. This study demonstrated that cisplatin-gemcitabine combination therapy resulted in a significant survival advantage without substantial additional toxicity. Gruenberger et al investigated the efficacy and safety of cetuximab in combination with gemcitabine and oxaliplatin (GEMOX) in a phase II study. The overall response rate was 63%, including 10% complete responses and 53% partial responses. Thirty percent of patients were able to undergo secondary curative resection after major response to therapy. The median overall survival was 15.2 months and the median progression-free survival was 8.8 months for all treated patients, including those who underwent secondary resection.
Sidebar: A Japanese phase II study compared gemcitabine plus S-1 (GS), an oral fluoropyrimidine, with S-1 alone in patients with advance biliary tract cancers. The median overall survival was 12.5 months vs 9 months and the median progression-free survival was 7.1 months vs 4.2 months in the GS and S-1 arms, respectively. Grade 3/4 adverse events were infrequent and reversible in both arms of the study. The authors suggest that the GS arm deserves to be compared with gemcitabine plus cisplatin in a phase III study (Morizane C et al: J Clin Oncol 30[4s]:abstract 255, 2012).
Targeted Therapies. There are several studies looking at the use of targeted biologic agents that have shown varying degrees of promise. Biliary tract cancers can overexpress EGFR and VEGF. Therefore, a multicenter phase II study by Lubner et al was done with daily erlotinib and bevacizumab given every 2 weeks in patients with unresectable cholangiocarcinoma or gallbladder carcinoma. Median overall survival was 9.9 months and time to progression was 4.4 months. Partial response was seen in 12% of the patients and stable disease was seen in 51% of patients. The partial response and stable disease rate of 64% is comparable to that for gemcitabine-based regimens. Molecular analysis showed that KRAS mutants are less likely to respond to erlotinib therapy than are other tumor types. Few grade 3/4 adverse events were seen with this combination of drugs. MEK has been studied as potential target given the important role of the RAS/RAF/MEK cascade in tumor formation. Selumetinib is a potent, oral small-molecule inhibitor of MAPK and MEK1/2. A multicenter phase II study by Bekaii-Saab et al was done with selumetinib in patients with advanced biliary tract cancers. Twelve percent of patients had an objective response. However, 68% of patients experienced stable disease, with 56% having prolonged stable disease for more than 16 weeks. The median progression-free survival was 3.7 months and the median overall survival was 9.8 months. Toxicities were mild, with rash and xerostomia being most common.
Sidebar: A phase II study was conducted using a combination of sorafenib and erlotinib in patients with advanced gallbladder cancer or cholangiocarcinoma. Eligible patients were given continuous sorafenib 400 mg PO twice daily and erlotinib 100 mg PO daily. This was a two-stage study with the primary endpoint being improvement in progression-free survival. Only 7% had a partial response and 27% had stable disease. The median progression-free survival was 2 months and the median overall survival was 6 months. The study failed to meets its primary endpoint and was terminated (El-Khoueiry AB et al: J Clin Oncol 30[s]:abstract 4113, 2012).
Hepatic arterial chemotherapy. There is limited experience with hepatic arterial chemotherapy for biliary tract neoplasms, but there are case reports of responses to floxuridine in the literature.
Treatment recommendations. In the absence of a clinical trial, patients should be offered gemcitabine with cisplatin because of the survival advantage seen with this combination.
SUGGESTED READING
On Hepatocellular Carcinoma
Abou-Alfa GK, Johnson P, Knox JJ, et al: Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: A randomized trial. JAMA 304:2154–2160, 2010.
Andolino DL, Johnson CS, Maluccio M, et al: Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 81:e447–e453, 2011.
Bhoori S, Schiavo M, Russo A, Mazzaferro V: First-line treatment for hepatocellular carcinoma: Resection or transplantation? Transplant Proc 39:2271–2273, 2007.
Cheng AL, Kang YK, Chen Z, et al: Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10:25–34, 2009.
Cheng BQ, Jia CQ, Liu CT, et al: Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: A randomized controlled trial. JAMA 299:1669–1677, 2008.
Finn RS, Kang YK, Mulcahy M, et al: Phase II, open-label study of brivanib as second-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res 18:2090–2098, 2012.
Gupta S, Bent S, Kohlwes J: Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C: A systematic review and critical analysis. Ann Intern Med 139:46–50, 2003.
Imamura J, Tateishi R, Shiina S, et al: Neoplastic seeding after radiofrequency ablation for hepatocellular carcinoma. Am J Gastroenterol 103:3057–3062, 2008.
Llovet J, Bruix J: Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 37:429–442, 2003.
Llovet JM, Ricci S, Mazzaferro V, et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390, 2008.
Marrero JA, Lencioni R, Kudo M, et al: Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma and its Treatment with Sorafenib (GIDEON) second interim analysis in more than 1,500 patients: Clinical findings in patients with liver dysfunction. J Clin Oncol 29(s):abstract 4001.
Mok TS, Yeo W, Yu S, et al: An intensive surveillance program detected a high incidence of hepatocellular carcinoma among hepatitis B virus carriers with abnormal alpha-fetoprotein levels or abdominal ultrasonography results. J Clin Oncol 23:8041–8047, 2005.
Morimoto M, Numata K, Kondou M, et al: Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: A randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer 116:5452–5460, 2010.
Salem R, Lewandowski RJ, Kulik L, et al: Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 140:497–507, 2011.
Sangro B, Carpanese L, Cianni R, et al: Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona Clinic Liver Cancer stages: A European evaluation. Hepatology 54:868–878, 2011.
Thomas MB, Jaffe D, Choti MM, et al: Hepatocellular carcinoma: Consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol 28:3994–4005, 2010.
Varela M, Real MI, Burrel M, et al: Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics. J Hepatol 46:474–481, 2007.
Yao FY, Bass NM, Ascher NL, Roberts JP: Liver transplantation for hepatocellular carcinoma: Lessons from the first year under the Model of End-Stage Liver Disease (MELD) organ allocation policy. Liver Transpl 10:621–630, 2004.
Zhang BH, Yang BH, Tang ZY: Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 130:417–422, 2004.
On Gallbladder Tumors
Cho SY, Kim SH, Park SJ, et al: Adjuvant chemoradiation in gallbladder cancer. J Surg Oncol 102:87–93, 2010.
Patt YZ, Hassan MM, Aguayo A, et al: Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer 101:578–586, 2004.
Takada T, Amano H, Yasuda H, et al: Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer 95:1685–1695, 2002.
Wang SJ, Lemieux A, Kalpathy-Cramer J, et al: Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. J Clin Oncol 29:4627–4632, 2011.
On Bile Duct Tumors
Alberts SR, Al-Khatib H, Mahoney MR, et al: Gemcitabine, 5-fluorouracil, and leucovorin in advanced biliary tract and gallbladder carcinoma: A North Central Cancer Treatment Group phase II trial. Cancer 103:111–118, 2005.
Bekaii-Saab T, Phelps MA, Li X, et al: Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol 29:2357–2363, 2011.
Cheng Q, Luo X, Zhang B, et al: Predictive factors for prognosis of hilar cholangiocarcinoma: Postresection radiotherapy improves survival. Eur J Surg Oncol 33:202–207, 2007.
Gruenberger B, Schueller J, Heubrandtner U, et al: Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: A phase 2 study. Lancet Oncol 11:1142–1148, 2010.
Horgan AM, Amir E, Walter T, Knox JJ: Adjuvant therapy in the treatment of biliary tract cancer: A systematic review and meta-analysis. J Clin Oncol 30:1934–1940, 2012.
Knox JJ, Hedley D, Oza A, et al: Combining gemcitabine and capecitabine in patients with advanced biliary cancer: A phase II trial. J Clin Oncol 23:2332–2338, 2005.
Lubner SJ, Mahoney MR, Kolesar JL, et al: Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: A phase II Consortium study. J Clin Oncol 28:3491–3497, 2010.
Rea DJ, Heimbach JK, Rosen CB, et al: Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg 242:451–461, 2005.
Valle J, Wasan H, Palmer DH, et al: Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–1281, 2010.
